Effects of Cochlear Trauma on BDNF Expression in Guinea Pig Cochlear Nucleus and Inferior Colliculus
Received: 11-Dec-2013 / Accepted Date: 08-Jan-2014 / Published Date: 16-Jan-2014 DOI: 10.4172/2161-119X.S3-006
Abstract
Hearing loss caused by cochlear damage results in a variety of plastic changes in the central auditory pathways. One of these is hyperactivity, i.e. increased spontaneous firing rates, which may be involved in the generation of tinnitus, a phantom auditory sensation. The mechanism behind this synaptic plasticity is still uncertain but there may be a role for Brain Derived Neurotrophic Factor (BDNF). The expression of BDNF is activity dependent and BDNF can modulate synaptic plasticity leading to changes in excitability. In the present study we investigated the effects of two different types of cochlear trauma, mechanical and acoustic, at two different time points after trauma, on 1) peripheral hearing loss, 2) hyperactivity in inferior Colliculus (IC) and 3) BDNF protein expression in Cochlear Nucleus (CN) and IC of guinea pigs. BDNF protein expression was determined using ELISA. Although there was no significant difference in the amount of hearing loss between acoustic trauma and mechanical trauma animals, single neuron recordings showed higher levels of hyperactivity after mechanical trauma than after acoustic trauma at two weeks post-recovery from cochlear trauma.In addition, results showed an increase of BDNF levels in the ipsilateral CN and contralateral IC at 2 weeks after mechanical but not after acoustic trauma. BDNF levels recovered to sham control levels in both structures at 6 weeks after cochlear trauma even though hyperactivity remained higher compared to sham surgery animals at the same timepoint. The results suggest a possible time dependent role for BDNF in modulating synaptic plasticity and excitability after mechanical trauma to the cochlea.
Keywords: Inferior colliculus, Hearing loss, Tinnitus, Guinea pig,Compound action potential, BDNF
Introduction
Trauma to the cochlea results in a reduced sensitivity to sound, but also in a variety of plastic changes in the central nervous system [1,2]. Changes in the central auditory pathway after hearing loss have been described using many different animal models and include changes in tonotopic maps, increased synchronous firing patterns and increased spontaneous firing rates [3-11]. These changes in central neural activity after cochlear trauma have been suggested to play a role in the development of tinnitus, a phantom auditory sensation that is commonly associated with the presence of hearing loss [12-14].
Increased spontaneous firing rates (hyperactivity) in the central pathways after cochlear trauma are thought to be the result of changes in intrinsic neuronal excitability [10,15] and in relative levels of inhibitory and excitatory neurotransmission as several studies on animal models of hearing loss have shown changes in the expression of genes and proteins related to GABAergic, glycinergic and glutamatergic neurotransmission throughout the auditory pathway [10,15-22].
Interestingly, the reported changes in gene and protein expression differ between these various studies and this may be dependent on the time-points investigated after cochlear trauma and on the type of trauma used [10,15,23,24]. This suggests that factors other than a loss of input from the cochlea may modulate ongoing plasticity for some time after cochlear trauma. Brain Derived Neurotrophic Factor (BDNF) is a potential factor because it is known to regulate synaptic plasticity and neuronal excitability. BDNF gene expression is under the control of neuronal activity and a constant factor in any cochlear trauma is a prolonged reduction of primary afferent activity posttrauma [25,26]. Moreover, BDNF is a regulator of glutamatergic and GABAergic activity [27,28]. Two earlier studies investigating BDNF protein expression used different types of cochlear trauma, cochlear ablation and acoustic trauma, and reported decreased and increased levels of BDNF in Inferior Colliculus (IC), respectively [29,30]. These results support the possible role for BDNF in driving different plastic changes depending on the type of cochlear trauma.
The present study investigates the role of BDNF in central plasticity after cochlear trauma. We directly compared the effects of two types of cochlear trauma, mechanical and acoustic, at two different time points after trauma, on peripheral hearing loss, hyperactivity in IC and BDNF protein expression in Cochlear Nucleus (CN) and IC. We used these two different types of cochlear trauma because although they both result in a reduction of primary afferent activity in the long-term, the initial effect of the trauma is distinctly different. Acoustic trauma will initially cause a transient large increase in driven activity (soundevoked) followed by a reduction in activity whereas mechanical trauma results in an immediate loss of cochlear sensitivity.
Experimental Procedures
Animals
Twenty-four adult pigmented guinea pigs of either sex were used, weighing between 245 and 385 g at the time of recovery surgery. These animals were used to create 6 experimental groups (n=4 each), consisting of three different treatments (sham, acoustic trauma and mechanical trauma) at 2 different recovery times (2 weeks or 6 weeks). All experimental protocols were approved by the Animal Ethics Committee of The University of Western Australia.
Anaesthesia and surgery
For recovery experiments during which the acoustic and mechanical trauma were performed as well as the initial baseline CAP recordings, animals were injected with 0.1 ml atropine sulphate (0.6 mg/ml) subcutaneously, followed by an intraperitoneal injection of Diazepam (5 mg/kg), and an intramuscular injection of Hypnorm 20 minutes later (0.315 mg/ml fentanyl citrate and 10 mg/ml fluanisone; 1 ml/kg). Absence of the foot withdrawal reflex was used to ascertain deep anaesthesia, after which animals were placed on a heating blanket in a soundproof room and the head mounted in hollow earbars. A small opening was made in the bulla on the left side and an insulated silver wire was placed on the round window in order to record a Compound Action Potential (CAP) audiogram (frequency range 4-24 kHz in 2 kHz steps) [31] using a closed sound system. All sound stimuli were presented through a ½” condenser microphone driven in reverse as a speaker (Bruel and Kjaer, type 4134). The system was calibrated using a 1/8” condensor microphone in place of the tympanic membrane and an absolute sound calibrator (Bruel and Kjaer type 4231). Pure tone stimuli were synthesized by a computer equipped with DIGI 96 soundcard connected to an analog/digital interface (ADI-9 DS, RME Intelligent Audio Solution). Sample rate was 96 kHz. The interface was driven by a custom-made computer program (Neurosound, MI Lloyd), which was also used to collect single neuron data during the nonrecovery experiment. CAP signals were amplified, filtered (100 Hz-3 kHz bandpass) and recorded with a second data acquisition system (Powerlab 4SP, AD Instruments). If cochlear thresholds were within the normal range the animal was randomly assigned to either a sham group, acoustic trauma or mechanical lesion group.
For acoustic trauma group the contralateral ear was blocked with plasticine and the animal was subjected to a continuous loud tone for 2 hours (10 kHz, 124 dB SPL). After the acoustic trauma CAP thresholds were again recorded to determine the magnitude of the immediate hearing loss.
For mechanical lesions, a small hole was hand drilled in the wall of the cochlea at the level of the basal turn and a glass micropipette electrode (tip diameter ~20 μm) filled with 150 mM KCI was inserted through the hole passing through scala tympani and the organ of Corti into scala media (signalled by the sudden appearance of a large positive potential between 80 and 100 mV). The pipette was then further advanced until it penetrated Reissner’s membrane (signalled by a drop in the positive voltage). The pipette was then withdrawn and a CAP audiogram obtained to establish loss of neural sensitivity. This procedure was repeated up to 3 times to ensure a substantial change in CAP thresholds, after which the hole in the cochlear wall was covered by a small piece of gelfilm.
Sham animals received no further treatment after the measurement of the CAP audiogram. Finally, in all animals the incision was sutured and buprenorphin (0.05 mg/kg subcutaneously) was given postoperatively as analgesic. Survival times were either 2 or 6 weeks.
For the final non-recovery experiments anaesthesia consisted of a subcutaneous injection with 0.1 ml atropine followed by an intraperitoneal injection of Nembutal (pentobarbitone sodium, 30 mg/kg) and a 0.15 ml intramuscular injection of Hypnorm. To maintain depth of anaesthesia animals were given 0.15 ml Hypnorm intramuscularly every hour and 15 mg/kg of Nembutal intraperitoneally every 2 hours. Animals were placed on a custom-made heating blanket thermostatically controlled to maintain animal rectal temperature at (38°C) in a sound proof room and artificially ventilated on carbogen (95% O2 and 5% CO2). After the animals were mounted in hollow earbars, the left and right cochleae were exposed and CAP audiograms were recorded on both sides with a silver wire placed on the round window as was done during the recovery experiments.
Inferior colliculus recording
Before single neuron recording paralysis was induced with 0.1 ml pancuronium bromide (2 mg/ml intramuscularly). The electrocardiogram was continuously monitored and heart rate never increased over pre-paralysis levels at any stage of the experiments. To obtain extracellular single neuron recordings in the CNIC a small craniotomy overlying the visual cortex was performed. A glass-insulated tungsten microelectrode [32] was advanced along the dorso-ventral axis through the cortex into the right central nucleus of the IC (CNIC) (contralateral to the exposed cochlea) using a stepping motor microdrive (Rapidsyn) and custom made controller. Electrode placement in the CNIC (about 2.5 to 3 mm ventral to the cortical surface) was indicated by the onset of strong sound-driven activity with a short latency and a systematic progression from low to high Best Frequencies (BF) with increasing depth. The craniotomy was covered with 5% agar in saline to improve mechanical stability. When a single unit was isolated its BF and threshold at BF were determined audio-visually and depth from the cortical surface was recorded. The Spontaneous Firing Rate (SFR) was measured for a period of 10s. During this measurement the polarization voltage of the speaker was turned off to avoid uncontrolled background noise from the sound delivery system.
Tissue collection
When the collection of electrophysiological data was finalized (either when we had recorded from approximately 100 neurons or when the ECG deteriorated (>350 ms intervals)), animals were decapitated and their brains rapidly removed in ice-cold phosphate-buffered saline. Both the left and right CN and IC were removed quickly using either a sharp scalpel or fine scissors, and then transferred into 1.5 ml RNasefree tubes. The samples were immediately stored at -80°C until further treatment.
ELISA analysis
Tissues were homogenized in 100 mM PIPES pH 7, 500 mM NaCl, 0.2% Triton X-100, 0.1% NaN3, 2 mM EDTA, 10 μM leupeptin, 0.3 μM aprotinin, and 1 μM pepstatin (pH 7 [33]) using syringe and needle combined with sonication. Subsequently, samples were centrifuged at 3,320×g for 1 hour and supernatants were stored at -80°C until used. BDNF protein levels were measured using the Chemi Kine TM Brain Derived Neurotrophic Factor (BDNF) Sandwich ELISA Kit (Merck Millipore) following manufacturer´s instructions. Briefly, samples (run in duplicate) were incubated on a 96 well plate pre-coated with mouse monoclonal antibody against human BDNF. The captured BDNF is then detected by a BDNF specific, biotin conjugated, mouse monoclonal antibody followed by addition of streptavidin-HRP, substrate and detection of optical density at 580 nm. BDNF concentrations were calculated from a standard curve and then normalized relative to total protein concentrations, which were determined using a micro BCA Protein Assay Kit (Pierce). Final values are expressed as ng BDNF/g protein.
Data analysis
Statistical analysis of CAP threshold changes after each treatment and at each frequency was performed using a Kruskall-Wallis test and a Dunn’s multiple comparisons post-test. For statistical comparison of the single neuron’s spontaneous firing rates in the CNIC between the different frequency regions Kruskall-Wallis test and a Dunn’s multiple comparison post-test were used. ELISA results were analysed using ANOVA with Bonferroni’s Multiple Comparison Tests. Statistical analysis was performed using GraphPad Prism.
Results
Peripheral auditory thresholds
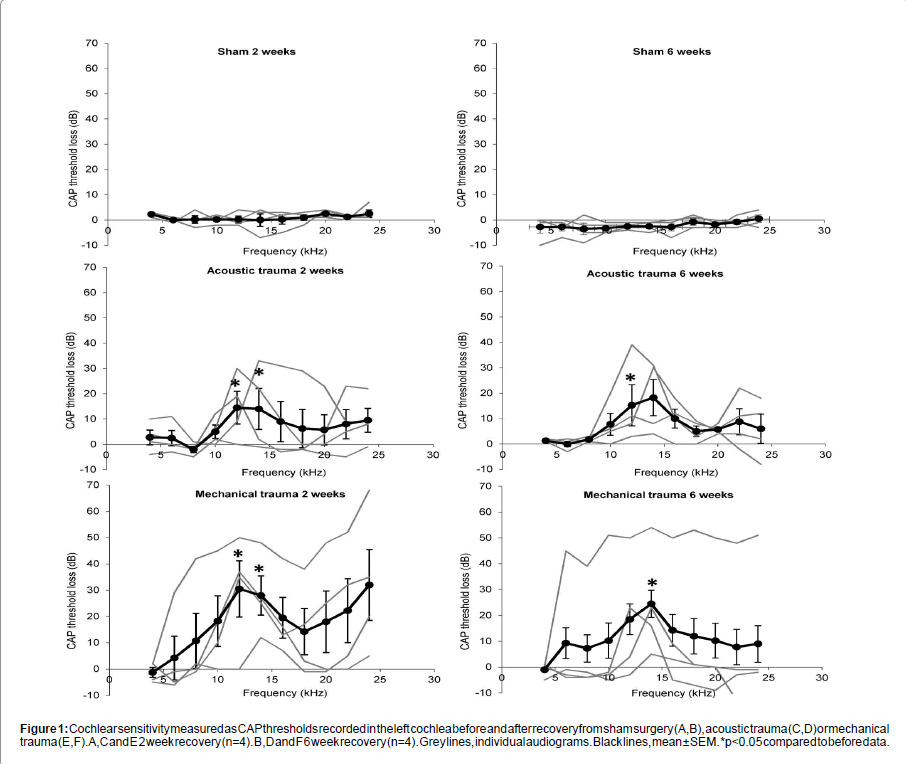
Sham surgery had no significant effect on peripheral thresholds at either 2 or 6 weeks recovery (Figures 1A and 1B). Acoustic and mechanical trauma both resulted in hearing loss after recovery, but there was large variation in the patterns of hearing loss between the individual animals (Figures 1C-1F). Statistical comparisons of the groups showed that 2 weeks after acoustic and mechanical trauma there was a significant threshold loss at 12 kHz and 14 kHz (p<0.05 and p<0.01 respectively) (Figures 1C and 1E). After six weeks recovery there was a significant threshold loss at 12 kHz after acoustic trauma (p<0.05) and at 14 kHz after mechanical trauma (p<0.05) (Figures 1D and 1F). There were no statistically significant differences between the hearing loss caused by mechanical and acoustic trauma after either 2 weeks or 6 weeks recovery.
Spontaneous firing rates in inferior colliculus
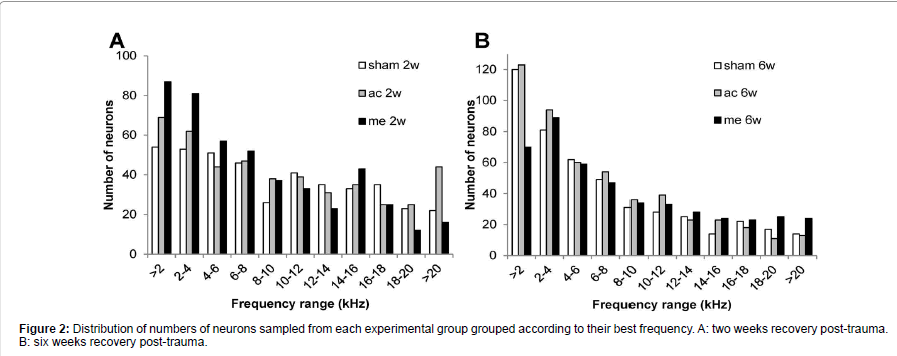
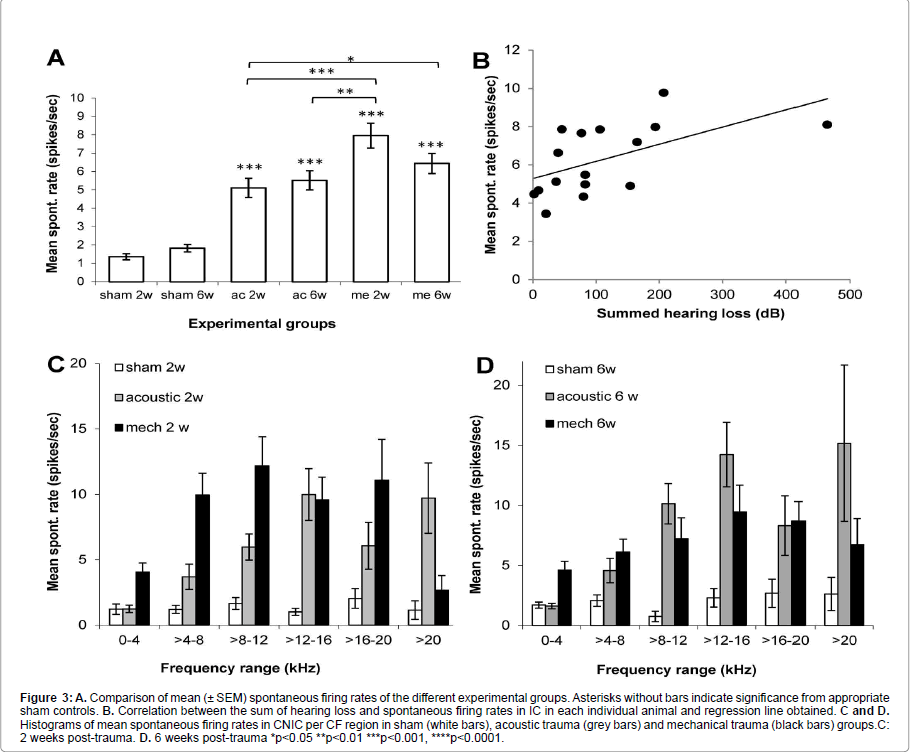
In each animal, spontaneous firing rates were collected from between 78 and 138 single CNIC neurons (mean 116 ± 2.7). As shown in Figure 2 the BF distribution of neurons sampled was very similar between the different groups. Figure 3A shows that in all 4 trauma groups mean SFRs recorded in the central nucleus of the inferior colliculus were statistically significantly increased compared to their respective sham surgery group (p<0.001). In addition, mechanical trauma resulted, after 2 weeks recovery, in higher mean spontaneous activity compared to acoustic trauma either after 2 weeks (p<0.001) or 6 weeks recovery (p<0.01). After 6 weeks recovery from mechanical trauma levels of spontaneous activity were not different from the data obtained 2 weeks after mechanical trauma or from data 6 weeks after acoustic trauma, but did show a significant elevation compared to 2 weeks recovery from acoustic trauma (p<0.05) (Figure 3A).
Figure 1: Cochlear sensitivity measured as CAP thresholds recorded in the left cochlea before and after recovery from sham surgery (A,B), acoustic trauma (C,D) or mechanical trauma (E,F). A,C and E 2 week recovery (n=4). B,D and F 6 week recovery (n=4). Grey lines, individual audiograms. Black lines, mean ± SEM. *p<0.05 compared to before data.
Figure 3:A. Comparison of mean (± SEM) spontaneous firing rates of the different experimental groups. Asterisks without bars indicate significance from appropriate sham controls. B. Correlation between the sum of hearing loss and spontaneous firing rates in IC in each individual animal and regression line obtained. C and D. Histograms of mean spontaneous firing rates in CNIC per CF region in sham (white bars), acoustic trauma (grey bars) and mechanical trauma (black bars) groups.C: 2 weeks post-trauma. D. 6 weeks post-trauma *p<0.05 **p<0.01 ***p<0.001, ****p<0.0001.
The correlation between the amount of hearing loss and spontaneous firing rates in IC was also investigated. An index of hearing loss was derived by summing every threshold change at each frequency of the CAP audiogram for each individual animal and plotting these values as a function of mean spontaneous firing rate. Figure 3B shows the weak (r2=0.3176) but statistically significant correlation (p=0.023) found, illustrating that there is a tendency for increased hearing loss to correlate with increased spontaneous firing rates in IC.
To further investigate the distribution of hyperactivity over the frequency regions of the CNIC,neurons were binned into six groups based on their BFs. Figures 3C and 3D show the result of this analysis at 2 and 6 weeks post-trauma, respectively. In sham animals spontaneous activity is low, irrespective of frequency range. In agreement with our previous reports the largest increases in spontaneous firing rates are observed in the frequency regions of peripheral hearing loss (Figure 1) [15,34,35]. In the case of mechanical lesions only, at two weeks post-recovery significant increases in spontaneous firing rate were also observed in lower frequency regions (Figure 3C).
ELISA analysis
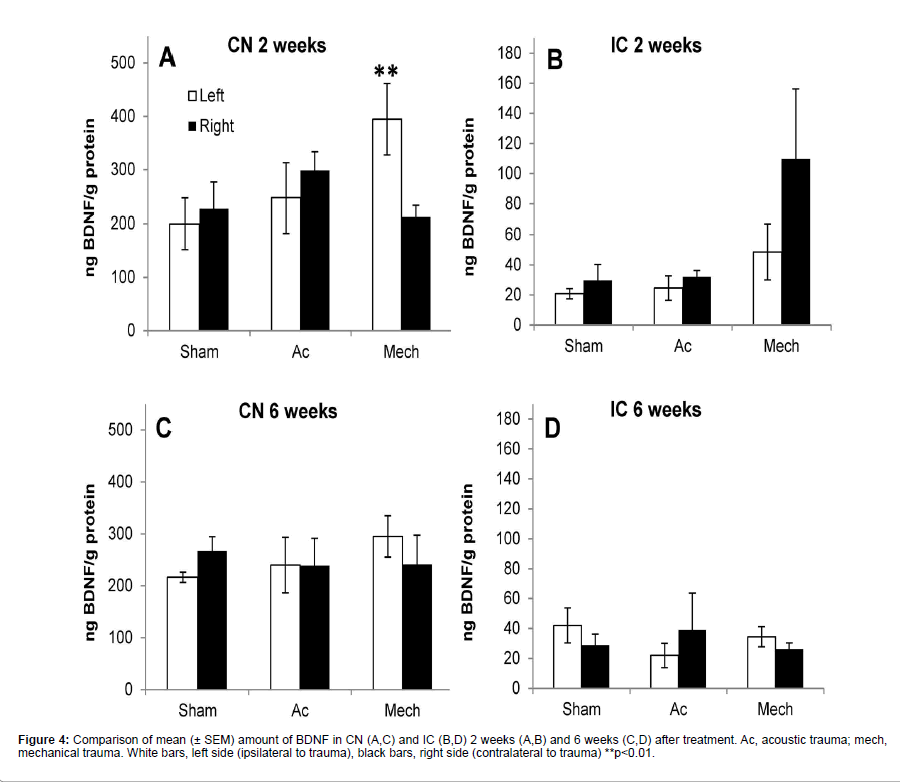
Figure 4 shows the results of ELISA on CN and IC samples at 2 and 6 weeks after surgery. Baseline levels of BDNF were much higher in CN compared to IC. Two weeks after recovery ANOVA showed an effect of treatment on CN samples (F(5,23)=4.125, p=0.0113). Post-hoc analysis showed a statistically significant effect between the left CN after sham surgery and the left CN after mechanical trauma (p<0.01; 83% increase) (Figure 4A). In the IC at 2 weeks post-treatment there was an increase of 270% in the amount of BDNF in the right IC after mechanical trauma compared to the sham control, however ANOVA failed to reach significance of treatment (F(5,19)=2.573, p=.0749) (Figure 4B).
Six weeks after treatment ANOVA showed a lack of significance of treatment on CN samples (F(5,25)=0.4746, p=0.7908) (Figure 4C) and on IC samples (F(5,19)=0.4023, p=0.8411) (Figure 4D). No significant correlation between BDNF levels and hearing loss or hyperactivity was found.
Discussion
The present study shows different effects on BDNF protein expression in the central auditory pathway depending on the type of cochlear trauma and the recovery time after trauma.
Both acoustic and mechanical trauma resulted in a notch–like CAP sensitivity loss that was largest at 12 and 14 kHz. For the acoustic trauma these frequencies of highest loss are just above the exposure frequency (10kHz), as has been demonstrated in previous studies of acoustic trauma [11,15,34,36,37] and which is due to the nonlinear properties of the basilar membrane at high intensities of sound [38,39].
Hyperactivity in CNIC was observed after both forms of trauma, as we have reported previously, although in our earlier studies only changes 1 week after mechanical lesion were investigated [10,15,34]. Hearing loss from different types of trauma has been shown to result in hyperactivity in IC and other auditory structures by many groups [5,6,9,40,41].
The hyperactivity after mechanical trauma was significantly higher than after acoustic trauma at 2 weeks post-trauma. This may be due to the magnitude of the peripheral hearing loss, as we have previously reported a correlation between magnitude of hearing loss and levels of hyperactivity, after two types of acoustic trauma with different exposure duration [11]. There was indeed a slightly larger peripheral threshold loss after mechanical trauma. In addition, correlation analysis confirmed a relationship between hearing loss magnitude and level of hyperactivity. However, the different magnitude of hyperactivity may also be the result of different types of damage to the cochlea. We have shown that the type of acoustic trauma employed in this study does not result in loss of inner or outer hair cells in the frequency region of the cochlea that corresponds to the hyperactive region in the CNIC [11]. On the other hand, mechanical trauma is likely to result in immediate localized outer and inner hair cell and associated nerve fibre damage [3,42]. It should be noted however that even without the loss of hair cells, there can be a specific loss of high threshold cochlear nerve fibres contacting the inner hair cells after acoustic trauma [43-45]. The difference in the type and number of nerve fibres degenerating after mechanical and acoustic trauma may well result in different central mechanisms leading to different levels of increased neural activity.
The main aim of this study was to investigate the effect of cochlear trauma on BDNF protein expression in central auditory pathway at different time points. A previous study using unilateral cochlear ablation in guinea pigs, showed an increase of BDNF protein in ipsilateral CN 3 days post-ablation, no change at 7 days and a further increase at 60 days post ablation. They also showed decreased BDNF protein expression in the contralateral IC at 3 and 7 days post-ablation with levels recovering to control levels after 60 days [29]. Another study showed increased levels of BDNF protein expression in IC six days after an acoustic trauma [30]. Although these studies suggest different effects on BDNF protein expression dependent on the method used to induce deafness and the time-point after the cochlear trauma, direct comparison is not possible. In the present study we were able to directly compare the effects of two different types of trauma and recovery times on BDNF protein expression.
Only mechanical, but not acoustic, trauma resulted in an increased expression of BDNF protein in the ipsilateral CN and contralateral IC. This pattern of increased BDNF protein expression is in agreement with the principal ascending pathway from the cochlea that received the trauma [46,47]. In addition, this effect appeared to be transient, present at two weeks post-trauma but not at 6 weeks post-trauma. This result is interesting in view of the fact that hyperactivity levels in CNIC were significantly higher after mechanical trauma than after acoustic trauma at two weeks, but not at 6 weeks post-trauma. This suggests a possible relationship between the increased hyperactivity and increased levels of BDNF protein, although it cannot be excluded that the two phenomena are not causally related. However, if they are related to each other, the hyperactivity could be the cause or the result of changes in BDNF protein expression. It is known that increased activity can stimulate BDNF gene expression [25,26] and so it may be that increased levels of central neural activity after mechanical trauma compared to acoustic trauma are sufficient to increase the levels of BDNF protein. The increased level of BDNF protein at 2 weeks could possibly upregulate inhibitory mechanisms [48,49], reducing the hyperactivity after mechanical trauma to levels similar to after acoustic trauma at 6 weeks (Figure 3).
An alternative hypothesis is that BDNF plays some causative role in the increased hyperactivity observed 2 weeks after mechanical trauma compared to the acoustic trauma. BDNF can cause axonal sprouting and synaptogenesis [50] and this could potentially lead to increased excitability in IC neurons and hence more activity. However, this seems unlikely in our model since at 6 weeks post-trauma, the level of hyperactivity after mechanical lesion is no longer different from the level of hyperactivity after acoustic trauma.
As suggested in the introduction, different gene and protein expression levels of BDNF after different types of cochlear trauma may lead to different expression patterns of genes associated with inhibitory and excitatory neurotransmission. BDNF serves a regulatory role in neuronal excitability through both transcriptional and posttranscriptional mechanisms [51]. In vitro studies using hippocampal neuron cultures and slices have shown that BDNF can up-regulate the density of GABAergic synapses and potentiate GABAergic transmission [48,49]. In addition, mice lacking activity-dependent BDNF gene expression showed levels of GABA release in barrel cortex that were significantly reduced, similar to levels seen in normal mice that were deprived of sensory inputs in postnatal life [52]. Interestingly, Tan et al. [30] described up-regulation of BDNF protein in IC 6 days after acoustic trauma which was accompanied by increased GABA protein expression in the same nucleus. It may therefore be that ongoing changes in BDNF protein expression regulate the balance of GABAergic and glutamatergic neurotransmission in central pathways after cochlear trauma.
References
- Liberman MC (1984) Single-neuron labeling and chronic cochlear pathology. I. Threshold shift and characteristic-frequency shift. Hear Res 16: 33-41.
- Liberman MC, Kiang NY (1984) Single-neuron labeling and chronic cochlear pathology. IV. Stereocilia damage and alterations in rate- and phase-level functions. Hear Res 16: 75-90.
- Robertson D, Irvine DR (1989) Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol 282: 456-471.
- Noreña AJ, Eggermont JJ (2005) Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci 25: 699-705.
- Seki S, Eggermont JJ (2003) Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res 180: 28-38.
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ (2008) Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res 86: 2564-2578.
- Finlayson PG, Kaltenbach JA (2009) Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res 256: 104-117.
- Kaltenbach JA, Zhang J, Afman CE (2000) Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res 147: 282-292.
- Komiya H, Eggermont JJ (2000) Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol 120: 750-756.
- Dong S, Mulders WH, Rodger J, Robertson D (2009) Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neuroscience 159: 1164-1174.
- Mulders WH, Ding D, Salvi R, Robertson D (2011) Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J Comp Neurol 519: 2637-2647.
- Axelsson A, Ringdahl A (1989) Tinnitus--a study of its prevalence and characteristics. Br J Audiol 23: 53-62.
- Eggermont JJ, Roberts LE (2004) The neuroscience of tinnitus. Trends Neurosci 27: 676-682.
- Salvi R, Lobarinas E, Sun W (2009) Pharmacological Treatments for Tinnitus: New and Old. Drugs Future 34: 381-400.
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D (2010) Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci 31: 1616-1628.
- Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM (1999) Detection of glutamate decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neuroscience 93: 1375-1381.
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, et al. (1999) Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience 93: 307-312.
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM (2000) GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res 147: 251-260.
- Mossop JE, Wilson MJ, Caspary DM, Moore DR (2000) Down-regulation of inhibition following unilateral deafening. Hear Res 147: 183-187.
- Asako M, Holt AG, Griffith RD, Buras ED, Altschuler RA (2005) Deafness-related decreases in glycine-immunoreactivelabeling in the rat cochlear nucleus. J Neurosci Res 81: 102-109.
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, et al. (2005) Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem 93: 1069-1086.
- Dong S, Rodger J, Mulders WH, Robertson D (2010) Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res 1342: 24-32.
- Marianowski R, Liao WH, Van Den Abbeele T, Fillit P, Herman P, et al. (2000) Expression of NMDA, AMPA and GABA(A) receptor subunit mRNAs in the rat auditory brainstem. I. Influence of early auditory deprivation. Hear Res 150:1-11.
- Kou ZZ, Qu J, Zhang DL, Li H, Li YQ (2013) Noise-induced hearing loss is correlated with alterations in the expression of GABAB receptors and PKC gamma in the murine cochlear nucleus complex. Front Neuroanat 7: 25.
- Castrén E, Zafra F, Thoenen H, Lindholm D (1992) Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A 89: 9444-9448.
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, et al. (2000) Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem 275:17269-17275.
- Matsumoto T, Numakawa T, Yokomaku D, Adachi N, Yamagishi S, et al. (2006) Brain-derived neurotrophic factor-induced potentiation of glutamate and GABA release: different dependency on signaling pathways and neuronal activity. Mol Cell Neurosci 31:70-84.
- Singh B, Henneberger C, Betances D, Arevalo MA, Rodriguez-Tebar A, et al. (2006) Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons. J Neurosci 26:7189-7200.
- Suneja SK, Yan L, Potashner SJ (2005) Regulation of NT-3 and BDNF levels in guinea pig auditory brain stem nuclei after unilateral cochlear ablation. J Neurosci Res 80: 381-390.
- Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, et al. (2007) Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience 145:715-726.
- Johnstone JR, Alder VA, Johnstone BM, Robertson D, Yates GK (1979) Cochlear action potential threshold and single unit thresholds. J AcoustSoc Am 65: 254-257.
- Merrill EG, Ainsworth A (1972) Glass-coated platinum-plated tungsten microelectrodes. Med BiolEng 10: 662-672.
- Szapacs ME, Mathews TA, Tessarollo L, Ernest Lyons W, Mamounas LA, et al. (2004) Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods 140: 81-92.
- Mulders WH, Robertson D (2009) Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience 164: 733-746.
- Robertson D, Bester C, Vogler D, Mulders WH (2013) Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hear Res 295: 124-129.
- Salvi RJ, Hamernik RP, Henderson D (1979) Auditory nerve activity and cochlear morphology after noise exposure. Arch Otorhinolaryngol 224: 111-116.
- Cody AR, Robertson D (1983) Variability of noise-induced damage in the guinea pig cochlea: electrophysiological and morphological correlates after strictly controlled exposures. Hear Res 9: 55-70.
- Cody AR, Johnstone BM (1981) Acoustic trauma: single neuron basis for the "half-octave shift". J AcoustSoc Am 70: 707-711.
- Sellick PM, Patuzzi R, Johnstone BM (1982) Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J AcoustSoc Am 72: 131-141.
- Kaltenbach JA, Afman CE (2000) Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res 140: 165-172.
- Vogler DP, Robertson D, Mulders WH (2011) Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci 31: 6639-6645.
- Robertson D, Johnstone BM, McGill TJ (1980) Effects of loud tones on the inner ear: a combined electrophysiological and ultrastructural study. Hear Res 2: 39-43.
- Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci 29: 14077-14085.
- Lin HW, Furman AC, Kujawa SG, Liberman MC (2011) Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol 12: 605-616.
- Furman AC, Kujawa SG, Liberman MC (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110: 577-586.
- Osen KK (1972) Projection of the cochlear nuclei on the inferior colliculus in the cat. J Comp Neurol 144: 355-372.
- Cant NB, Benson CG (2003) Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull 60: 457-474.
- Marty S, Wehrlé R, Sotelo C (2000) Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci 20: 8087-8095.
- Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, et al. (2005) BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex 15: 291-298.
- Kaplan GB, Vasterling JJ, Vedak PC (2010) Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol 21: 427-437.
- Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76: 99-125.
- Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, et al. (2011) A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci U S A 108: 12131-12136.
Citation: Mulders W, Rodger J, Albertsen M, Yates CG, Robertson D (2014) Effects of Cochlear Trauma on BDNF Expression in Guinea Pig Cochlear Nucleus and Inferior Colliculus. Otolaryngology S3:006. Doi: 10.4172/2161-119X.S3-006
Copyright: © 2014 Mulders W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14147
- [From(publication date): 1-2012 - Apr 23, 2024]
- Breakdown by view type
- HTML page views: 9710
- PDF downloads: 4437