Circadian Rhythm in Stroke: The Influence of Our Internal Cellular Clock on Cerebrovascular Events
Received: 03-Feb-2014 / Accepted Date: 28-Feb-2014 / Published Date: 03-Mar-2014 DOI: 10.4172/2161-0681.1000163
Abstract
The distinct temporal pattern of stroke occurrence in humans has been recognized for decades; yet, the reason underlying the temporal nature of stroke is not completely understood. Several exogenous factors such as seasonal variation, physical activity, diet and sleep/wake cycles can influence stroke occurrence. Furthermore, it has been increasingly recognized that there are several endogenous physiological functions such as blood pressure, autonomic nervous system activity, and coagulation that show temporal variance and ultimately influence susceptibility to stroke. It was long believed that the neurons within the Suprachiasmatic Nucleus (SCN) controlled all of the body’s circadian rhythm cycles serving as the “master clock”. However, circadian gene expression is inherent to almost every cell in the body, controlling cellular metabolism, and ultimately an organ’s susceptibility to injury. These new insights into the molecular mechanisms regulating circadian rhythmicity might help to explain the phenomenon of circadian variation in stroke occurrence.
Keywords: Circadian rhythm; Circadian rhythm signaling proteins; Ischemic stroke; Intracerebral hemorrhage; Subarachnoid hemorrhage
Introduction
The term “circadian rhythm” stems from the Latin expressions “circa” (about, around) and “dies” (day), describing the endogenous physiologic rhythmicity with an approximate duration of 24-hours that is inherent to all living organisms. It not only dictates the endogenous sleep/wake cycle, but also influences behavior and nearly every physiological function. This “internal clock” can be influenced by external factors such as light [1] or nutrition [2], making it a “diurnal” cycle synchronized to 24-hour alternating intervals of light and dark. The master clock in humans is known as the Suprachiasmatic Nucleus (SCN) located in the anterior hypothalamus, where the organism’s circadian rhythm originates. However, it has become recognized that almost every cell has a “clock”, regulating molecular and cellular functions guided by the same circadian rhythmicity that then affects metabolism, and ultimately susceptibility to stress or injury [3].
A circadian rhythm is generated at the molecular level by expression patterns of an array of genes, controlled by several transcription factors. The products of these genes in turn serve as repressors for the transcription factors, completing a negative feedback loop that keeps the cycle oscillating in a circadian manner [4]. The specific downstream targets of these clock genes have yet to be elucidated, but it seems that the target genes that are utilmately modulated are highly tissue specific [5].
Literature supports the concept of distinct circadian distribution patterns for the frequency of cerebrovascular events such as ischemic or hemorrhagic stroke [6-8]. While earlier studies focused on exogenous factors that influenced stroke frequency such as physical activity, food intake, stress or medication [9], researchers have become increasingly interested in the role that the internal clock might play in determining the onset and frequency of cerebrovascular events, as well as the severity of neuronal damage and clinical outcome. Furthermore, it is known that hemodynamic and cardiovascular parameters that influence the risk of cerebrovascular events, such as blood pressure, heart rate, and coagulation, follow circadian variability [10], and are controlled by the endogenous clock [11-13].
The first part of this review will focus on the clinical aspects of stroke and its striking circadian distribution. In the second part, we will review the current literature on how circadian rhythm is generated at a molecular level, followed by a discussion on how the molecular clock and circadian gene expression might be involved in the aforementioned circadian distribution of stroke. Finally, we will discuss the intriguing link between gasotransmitters Carbon Monoxide (CO), Nitric Oxide (NO), and regulation of the molecular clock.
Part 1: Circadian Rhythm in Stroke
Since the 1970s, there have been numerous reports linking circadian rhythm and stroke occurence [14]. Ischemic and hemorrhagic stroke can exhibit a bimodal frequency of ictal events [9]. When strokes of all types are considered collectively, the ictal event occurs more often in the morning and early afternoon hours [15,16]. Circadian periodicity is not only seen in the occurence of ictal events, but also fatality due to stroke. One study shows fatality to be higher when occuring in the morning as opposed to afternoon, even when adjusting for age, gender, and severity [17].
Ischemic stroke, intracebral hemorrhage, and subarachnoid hemorrhage are the three major types of stroke, each with its own pathophysiologic mechanisms and set of clinical characteristics. Often, these different stroke types have reportedly different circadian patterns when looked at separately.
Circadian rhythm in ischemic stroke
Ischemic Stroke (IS) events are caused by reduced blood flow to a region of the brain due to obstruction of an artery. Of the three major stroke classifications, IS is unique in that it is the only type to have maximal yearly ictal events during the same time period for all its subtypes.
IS is reported to occur with a maximal peak in the morning hours, and a second minor peak in the evening; this circadian pattern is independently associated with the occurrence of IS even when controlling for hypertension, diabetes, hyperlipidemia, smoking habits, previous vascular events, and treatment with anti-platelet agents or anticoagulant drugs [18,19]. Even when IS is divided into the subgroups of small artery or lacunar stroke, cardioembolic, large artery, and cryptogenic, the highest probability of ictus is still in the morning hours for each subtype of IS [20-22]. Unlike IS where incidence is greatest in the moring, studies of intracerebral hemorrhage show this to not hold true for all stroke types.
Circadian rhythm in intracerebral hemorrhage
Intracerebral Hemorrhage (ICH) is defined as an arterial bleed into the brain parenchyma. What is interesting about circadian rhythm in ICH is that patients with different demographics tend to show different circadian patterns of ictal events.
Unlike IS, ICH has a higher occurrence rate in the late afternoon, with rare observation of ictus at night [23]. This time of the day distribution pattern remains when only hypertensive ICH patients are considered as well as when ICH is subgrouped according to location of hemorrhage. However, differences in ictal onset emerge when biographics such as age and gender are considered. For example, one study found a single morning peak of ICH occurence in men 69 years of age or younger. In contrast, men over 70 as well as woman of all ages were shown to only have a single evening peak [23].
Whether or not one is sleeping during an ICH, it also seems to affect outcome. When ICH mortality was compared in patients who were asleep versus awake during the ictal event, the sleeping patients had a signficantly higher mortality. Not surprisingly, the hemorrhage volume in the sleeping group was significantly larger [24].
Clinical studies of ICH show the importance of normal 24-hour biological circadian rhythm in regulating physiologic functions such as Blood Pressure (BP) and Heart Rate (HR). In the hours prior to death due to ICH, the normal circadian oscillation pattern of these parameters was absent [25]. BP and HR also aid in predicting the prognosis of ICH patients that undergo neurosurgical intervention. When the BP and HR circadian rhythms remain normal for 24-hours postoperatively in hypertensive ICH, better clinical outcomes are observed [26].
ICH presents with a different pattern of ictus than that of the other type of hemorrhagic stroke: Subarachnoid hemorrhage. The overall peak of SAH events vary depending on the season.
Circadian rhythm in subarachnoid hemorrhage
Subarachnoid Hemorrhage (SAH) is bleeding into the subarachnoid space surrounding the brain due to vessel destruction. Unlike IS and ICH, SAH presents with different cerebrovascular event hours depending on the temperature.
SAH occurence is highest in the morning during the colder months and highest in the afternoon during the warmer months with a significant increase in frequency on Sunday (with Monday as a reference variable). Weekend-related changes in diet, alcohol consumption and physical activity have been suggested to explain this phenomenon [27]. Differences are found when SAH is divided into two subgroups: Aneurysmal SAH (aSAH) caused by an aneurysm rupture and non-aneurysmal SAH (naSAH). Aneurysmal SAH reportidly occur most often during the morning hours whereas no peak occurrence is reported in naSAH [28,29].
Each type of stroke presents with a uniqe circadian rhythm. This is not surprising as each category of stroke not only has a distinct clinical presentation, but also has a unique pathophysiology, as well as different risk factors. Various exogenous and endogenous factors show circadian oscillation, including blood pressure, pro-thromboembolic factors, autonomic nervous system regulation, and occurence of atrial fibrillation. These are considered to be potential factors in circadian rhythm of stroke ictus and each will be discussed below.
Exogenous and endogenous factors in stroke frequency
Seasonal variation: Seasonal variations seem to affect the risk of stroke. There is higher frequency of occurrence for embolic ischemic stroke, ICH, and SAH during the colder months [30-33]. This same seasonal pattern is seen in patients with putaminal and subcortical ICH [23]. ICH of undetermined origin shows no significant seasonal pattern [33].
SAH events peak in February with risk factors including low temperature on both the day prior to as well as day of onset as well as high barometric pressure on the onset day [34]. SAH events reportedly peak in colder months and dip in the warmer months for patients aged 59 years or younger. SAH seasonal variations are most significant in the morning hours regardless of patient age or season [35]. Why this seasonal variation in ictal events occurs is not yet completely understood and requires further research on the possible association between seasonal variation and stroke.
Pro-thromboembolic factors and autonomic nervous system activity: The pattern of IS events is potentially due to hypercoagulability that occurs with greater frequency in the early hours of the day [18]. Epinephrine, norepinephrine, and platelet aggregation are increased in the morning, approximately 90 minutes after assumption of the upright position [36]. Platelet aggregation increases significantly during the morning hours, but the increase is not significant when subjects remain supine and inactive [37]. This could explain the increased IS risk in the morning. Additionally, increased alpha-sympathetic vasoconstriction activity in the morning with a significantly higher basal forearm vascular resistance and lower blood flow further increases the risk of IS [38].
There have been recent reports showing that the circadian rhythm of hemostatic factors can have an effect on tissue Plasminogen Activator (tPA), a commonly used treatment for IS. Acute ischemic stroke due to middle cerebral artery occlusion treated with i.v. tPA has been shown to produce better clinical outcomes in patients treated during diurnal tPA administration (9 am-9 pm) compared to those treated during the nocturnal period (9 pm-9 am) [39]. In addition to pro-thrombotic factors and nervous system regulation, BP physiology patterns may be involved in causing the differences in stroke frequency. The role of BP variation in relation to ictal events will be discussed in the next section.
Blood pressure: Blood Pressure (BP) has circadian regularity and is an accepted risk factor for stroke. Changes in circadian rhythm are witnessed in stroke patients. A 2004 study found that normal diurnal variation in BP was abolished in a majority of acute stroke patients, where BP normally decreases by at least 10% in the evening hours [40]. There have also been circadian BP differences among ischemic stroke subsets [41]. Lacunar or small artery stroke is a subtype of IS that results from occlusion of an artery delivering blood to deep brain structures. Patients with lacunar strokes present with a mean decline in day-night systolic and diastolic BP of approximately 4 mmHg compared to non-lacunar infarction patients, who show no 24-hour nocturnal BP decrease [42].
Even though it is generally accepted that high BP is a risk factor for stroke, further studies are needed to determine whether changes in BP occur before and/or after an ictal event. Another widely accepted risk factor for stroke that is thought to have involvement in the circadian rhythm of ictal events is atrial fibrillation.
Atrial fibrillation: Atrial fibrillation is a cardiac arrhythma disorder leading to abnormal impulse conduction to the ventricular chambers of the heart. This heart rhythm disorder is accepted as a risk factor for stroke, and now there is evidence that some patients who present with disordered heart rhythm also show a temporal pattern of stroke occurence. One study in 2001 describes a distinct stroke frequency in atrial fibrillation patients, with the highest occurrence of stroke to be during general daylight hours, and the lowest occurrence to be during early morning hours [43]. Patients who report a single episode of cardioembolic acute stroke due to atrial fibrillation, show a higher incidence spike in the morning, and a second lower peak during the late afternoon [44]. A separate study finds that some chronic atrial fibrillation patients lose their diurnal variation in hypercoagulability along with the loss of diurnal variation in hemostatic markers: both coagulability and hemostatic factors abnormally remain in a hyperactive state in these patients [45]. In a recent publication, a specific relationship between atrial fibrillation and wake-up stroke was studied. An independent association between atrial fibrillation and wake-up ischemic stroke and transient ischemic stroke was reported, where there was a 3-fold increase in detecting a newly diagnosed atrial fibrillation in patients with wake-up cerebrovascular occurrences [46].
Part 2: Linking Circadian Patterns of Stroke to the Molecular Clock
Literature reports clearly show a wide range of both exogenous and endogenous factors to play a role in the circadian rhythm of stroke occurrence. More enlightenment into the role of the internal molecular clock inherent to all organs and cells could be key to understanding the role that these factors play in circadian susceptibility to ictal events. In the second part of this review, we will focus on how circadian rhythm is generated at the molecular level. This is followed by discussion on how circadian gene expression is involved in the circadian distribution of stroke, describing a possible mechanism responsible for this phenomenon.
The internal molecular clock
Circadian rhythm is generated at the molecular level by expression of several clock genes, the expression of which is controlled by corresponding transcription factors. Many genes associated with circadian rhythmicity have been identified, however, the general understanding is that there are eight core members that include: Period (Per1, 2 and 3), Cryptochrome (Cry1 and 2), and the transcription factors Clock, Arntl (Bmal1) and Npas2. Circadian oscillatory expression is controlled via an auto-regulatory feedback loop [4,47]. Expression of Per and Cry genes is driven by Bmal1/Clock or Bmal1/ Npas2 heterodimers binding to the corresponding E-box enhancer sites within the Per and Cry promotor regions. After translation, Per and Cry proteins heterodimerize and translocate into the nucleus, where they suppress the transcriptional activity of Clock, Bmal1 and Npas2, repressing their own transcription and closing a negative feedback loop that shows a circadian rhythmicity of gene expression. Protein level peaks for Per and Cry are delayed by approximately 6 hours compared to the corresponding mRNA peaks [48]. In addition, the following cofactors play an important role in the regulation and stabilization of the circadian molecular clock: Casein Kinase 1ε (CK1ε) that phosphorylates Per, leading to suppression of Clock- and Bmal1-DNA-binding [49] and Rev/Erb-α that represses transcription of Bmal1 [50]. A vast array of downstream genes is influenced in a circadian manner. Up to 5-10% of the entire genome varies with similar rhythmicity. However, this fraction of the genome varies in a highly tissue-specific manner [5,51-54] with little overlap between different organs, providing each tissue with an individual set of genes regulated in a circadian fashion.
This internal molecular clock is very accurate, even when external cues are missing. This has been shown in experiments with continuous darkness/light and forced phase shifts [55,56]. Meanwhile, the system is very dynamic in its ability to adjust to external cues in that it can reset its own “time” and synchronize to new environmental situations, especially in response to light (“phase delay” and “phase advance”) [1,57]. This adaptation ability has indeed proven to be very helpful in an evolutionary sense so to allow for adjustment to new ecological situations and the naturally occurring seasons. However, the internal molecular clock’s readiness to react to new external cues poses problems in situations of modern life that otherwise antagonize our “natural” internal rhythms, such as night shift work, continuous light exposure, and jet lag. Disruption of these innate rhythms increase the risk for several health problems including cardiac and metabolic diseases that ultimately lead to cerebrovascular events such as stroke [58-60].
Central vs. peripheral cellular (organ) clocks
The dogma of the SCN operating as the “master clock” controlling circadian rhythm of the whole body has been challenged by the discovery of peripheral clocks that exhibit endogenous and autonomous circadian rhythm [61-64]. Nearly all organs, except the testes, show a high expression of clock genes. An autonomous peripheral organ clock exists that is entrained and synchronized by SCN as the “pacemaker” dictated by external light and dark cues. Peripheral tissues will maintain their circadian gene expression profile ex vivo even without being entrained by the SCN [62,63,65]. However, the peripheral clocks eventually lose their ability to oscillate in a circadian manner after several cycles in vitro. In contrast, the SCN can maintain its periodicity for more than 30 days [62]. It remains an interesting and elusive subject as to how the SCN acts as the pacemaker and communicates with peripheral organs to synchronize their autonomous clocks. This most likely happens via circulating hormone levels [61,66], but the processes related to this synchronization are still poorly understood. Even in the brain, different regions exhibit their own autonomous rhythm that is most likely also synchronized by the SCN, but these regions show remarkable time shift capabilities in their clock gene expression profile [67-69].
Does stroke perturb the circadian clock?
On the one hand, sleep-related disorders such as Obstructive Sleep Apnea (OSA) are an independent stroke risk factor and have a significant influence on clinical outcome [70]. On the other hand, stroke itself can lead to disturbance in sleep-wake patterns [71], the treatment of which poses an immense challenge. Perturbation of sleep-wake cycles in stroke patients could easily be attributed to external factors such as light and noise exposure in the Intensive Care Unit (ICU) or hypnotic and sedative drug treatment [72]. However, experimental evidence suggests that the injurious event itself causes the circadian clock lose its original rhythm [73,74], altering the physiological circadian expression pattern in different brain regions. As a consequence, future clinical treatment strategies for stroke related sleeping disorders might have to be focused on re-setting the clock so as to improve sleep-wake behavior in these patients and improve clinical outcome. Additional evidence for injurious events to the brain that disrupt the molecular clock comes from clinical data showing that stroke patients lose their normal variation in BP (night-time dipping) [40], underlining the fact that the molecular clock is indeed “losing its beat” after neuronal injury.
Clock gene expression and cardiovascular dysfunction
The importance of clock genes for an organism’s homeostasis is underscored by the fact that mice deficient in different clock genes present with a vast array of pathological phenotypes. Mice deficient in Bmal1 suffer from disturbed sleeping behavior, liver and kidney dysfunction [75], metabolic impairment [76], musculosceletal weakness [77], arthritis [78] and eventually premature death [79]. Furthermore, Bmal1 and also Clock deficient mice suffer from cardiomyopathy [80], lack of a normal diurnal variation in BP and HR, endothelial dysfunction, and pathological vascular remodeling [11]. Vascular endothelial dysfuntion has also been confirmed for Per2 deficient mice [81]. Per2 expression has been shown to follow a circadian pattern in the heart [82]. Moreover, Per2 is upregulated following cardiac ischemic injury, exogenous induction of Per2 exerts significant protection against cardiac ischemic injury, and Per2 deficient mice show aggravated cardiac damage [82].
Experimental and clinical studies have shown that the function of the coagulation system undergoes dramatic changes throughout the course of a day [9,10]. This adds to the variable susceptibility to thrombotic or hemorrhagic events during the circadian cycle. In addition, physiological circadian BP changes are also under the control of clock gene expression. Mice with SCN ablation or loss in different clock genes lose their circadian BP variation [12], leading to pathological phenotypes that can trigger cerebrovascular injury.
Neuronal clock gene expression and neuronal injury
The aforementioned reports lead us to conclude that: 1.) disturbance of normal clock gene expression increases susceptibility to stroke and 2.) Different times of the day dictate a high or low susceptibility to cerebrovascular events depending on the actual expression level of the clock genes and that this exists even in organisms with normal clock gene expression patterns.
The brain might exhibit varying degrees of susceptibility to stroke depending on clock gene rhythmicity within the neuronal cells themselves. Since circadian rhythm gene expression may be differentially regulated in different brain regions [68,69], the susceptibility of the brain to injury might even vary within different regions of the brain. Some experimental data supports the idea that neuronal expression of circadian rhythm genes directly influences susceptibility to injury. One study reports that neuronal apoptosis in the hippocampus after ischemic injury is dependent on the time of the day and the quantity of Per expressed [73]. Additionally, ischemic neuronal injury at time points where Per1 expression is low lead to increased neuronal apoptosis. Knock-out studies with mice lacking Per1 revealed that these mice are more susceptible to neuronal cell death following ischemic injury [83]. In traumatic brain injury, Per2 expression in the hippocampus and the SCN is upregulated as early as 4-8 hours after injury [74]. The expression pattern of circadian rhythm genes and their potential protective role following other types of neuronal injury such as intracerebral or subarachnoid hemorrhage has not been explored.
Clock genes and gasotransmitters
Npas2, as Bmal1, can heterodimerize with Clock to control expression of Per2 and other clock genes. The DNA-binding activity of this transcription factor is modulated by Carbon Monoxide (CO) [84]. More recently, Clock and Rev-erbα activity has been associated with heme-binding properties that enable modulation by gasotransmitters including CO and Nitric Oxide (NO) [85,86]. The binding of these gases to the heme moiety in Rev/Erb-α likely influences the transcriptional activity of Clock and the suppressive function of Rev/Erb-α on Bmal1 transcription. Indeed a recent report by our laboratory shows that CO requires Rev/Erb-α to drive cellular repair [87]. This adds to the complexity of clock gene control since regulation through volatile gasotransmitters like CO and NO are likely to take place very rapidly, regardless of cellular borders. Exogenous application of these gaseous molecules might emerge as a useful tool for re-setting a perturbed internal clock and eventually improving clinical outcome after both neuronal and peripheral organ injury.
Conclusion
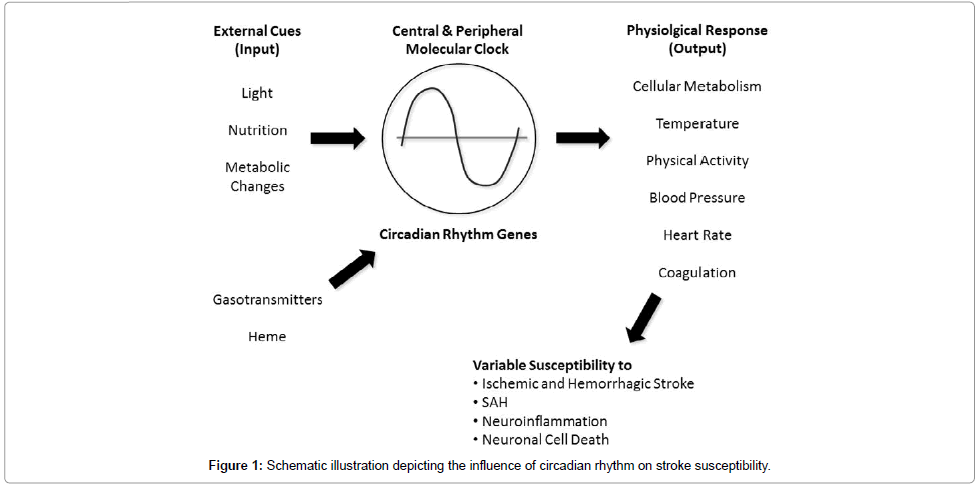
The circadian frequency pattern of stroke occurrence has long been acknowledged. There is strong evidence that in addition to exogenous factors, the circadian oscillation in cardiovascular function, coagulation and cellular metabolism dictates this variability. Central to the increased susceptibility is the molecular clock and when in the circadian cycle the insult or stress occurs. Figure 1 depicts the prospective link between the molecular clock and neuronal injury in stroke occurrence. With an understanding of how the rhythm of the clock influences susceptibility to pathophysiology, potential therapeutic approaches can then be designed and implemented.
Acknowledgements
The article has been funded and supported by the grants NIH K08NS078048 to KAH, and DFG SCHA1838/2-1 to NS.
References
- Best JD, Maywood ES, Smith KL, Hastings MH (1999) Rapid resetting of the mammalian circadian clock. J Neurosci 19: 828-835.
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291: 490-493.
- Bargiello TA, Jackson FR, Young MW (1984) Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312: 752-754.
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, et al. (2000) Interacting molecular loops in the mammalian circadian clock. Science 288: 1013-1019.
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, et al. (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307-320.
- Muller JE, Tofler GH, Stone PH (1989) Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79: 733-743.
- Tsementzis SA, Gill JS, Hitchcock ER, Gill SK, Beevers DG (1985) Diurnal variation of and activity during the onset of stroke. Neurosurgery 17: 901-904.
- Lavery CE, Mittleman MA, Cohen MC, Muller JE, Verrier RL (1997) Nonuniform nighttime distribution of acute cardiac events: a possible effect of sleep states. Circulation 96: 3321-3327.
- Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, et al. (2005) Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagic events. ChronobiolInt 22: 417-453.
- Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, et al. (2008) Genetic components of the circadian clock regulatethrombogenesis in vivo. Circulation 117: 2087-2095.
- Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, et al. (2009) Vascular disease in mice with a dysfunctional circadian clock. Circulation 119: 1510-1517.
- Rudic RD (2009) Time is of the essence: vascular implications of the circadian clock. Circulation 120: 1714-1721.
- Rudic RD, Fulton DJ (2009) Pressed for time: the circadian clock and hypertension. J ApplPhysiol (1985) 107: 1328-1338.
- Marshall J (1977) Diurnal variation in occurrence of strokes. Stroke 8: 230-231.
- Elliott WJ (1998) Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 29: 992-996.
- Pardiwalla FK, Yeolekar ME, Bakshi SK (1993) Circadian rhythm in acute stroke. J Assoc Physicians India 41: 203-204.
- Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, et al. (2012) Is there any circadian variation consequence on acute case fatality of stroke? Takashima Stroke Registry, Japan (1990-2003). ActaNeurolScand 125: 206-212.
- Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, et al. (2002) Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol 59: 48-53.
- Argentino C, Toni D, Rasura M, Violi F, Sacchetti ML, et al. (1990) Circadian variation in the frequency of ischemic stroke. Stroke 21: 387-389.
- Marsh EE 3rd, Biller J, Adams HP Jr, Marler JR, Hulbert JR, et al. (1990) Circadian variation in onset of acute ischemic stroke. Arch Neurol 47: 1178-1180.
- Chaturvedi S, Adams HP Jr, Woolson RF (1999) Circadian variation in ischemic stroke subtypes. Stroke 30: 1792-1795.
- Marler JR, Price TR, Clark GL, Muller JE, Robertson T, et al. (1989) Morning increase in onset of ischemic stroke. Stroke 20: 473-476.
- Inagawa T (2003) Diurnal and seasonal variations in the onset of primary intracerebral hemorrhage in individuals living in Izumo City, Japan. J Neurosurg 98: 326-336.
- Nagakane Y, Miyashita K, Nagatsuka K, Yamawaki T, Naritomi H (2006) Primary intracerebral hemorrhage during asleep period. Am J Hypertens 19: 403-406.
- Guan JW, Chen MJ, Li H, Liu YY, You C, et al. (2011) Characteristics of circadian rhythm in patients with intracerebral hemorrhage before death. Neurosciences (Riyadh) 16: 340-346.
- Guan J, Ding Y, Liu Y, Li Y, Liu Y, et al. (2009) Circadian effects on outcome following surgery for intracerebral hemorrhage in humans? Brain Res 1258: 78-85.
- Feigin VL, Anderson CS, Rodgers A, Bennett DA (2002) Subarachnoid haemorrhage occurrence exhibits a temporal pattern - evidence from meta-analysis. Eur J Neurol 9: 511-516.
- Miranpuri AS, Aktüre E, Baggott CD, Miranpuri A, Uluç K, et al. (2013) Demographic, circadian, and climatic factors in non-aneurysmal versus aneursymal subarachnoid hemorrhage. ClinNeurolNeurosurg 115: 298-303.
- Temes RE, Bleck T, Dugar S, Ouyang B, Mohammad Y, et al. (2012) Circadian variation in ictus of aneurysmal subarachnoid hemorrhage. Neurocrit Care 16: 219-223.
- Kelly-Hayes M, Wolf PA, Kase CS, Brand FN, McGuirk JM, et al. (1995) Temporal patterns of stroke onset. The Framingham Study. Stroke 26: 1343-1347.
- Nyquist PA, Brown RD Jr, Wiebers DO, Crowson CS, O'Fallon WM (2001) Circadian and seasonal occurrence of subarachnoid and intracerebral hemorrhage. Neurology 56: 190-193.
- Inagawa T, Takechi A, Yahara K, Saito J, Moritake K, et al. (2000) Primary intracerebral and aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Part I: incidence and seasonal and diurnal variations. J Neurosurg 93: 958-966.
- Passero S, Reale F, Ciacci G, Zei E (2000) Differing temporal patterns of onset in subgroups of patients with intracerebral hemorrhage. Stroke 31: 1538-1544.
- Abe T, Ohde S, Ishimatsu S, Ogata H, Hasegawa T, et al. (2008) Effects of meteorological factors on the onset of subarachnoid hemorrhage: a time-series analysis. J ClinNeurosci 15: 1005-1010.
- Inagawa T (2002) Seasonal variation in the incidence of aneurysmal subarachnoid hemorrhage in hospital- and community-based studies. J Neurosurg 96: 497-509.
- Brezinski DA, Tofler GH, Muller JE, Pohjola-Sintonen S, Willich SN, et al. (1988) Morning increase in platelet aggregability. Association with assumption of the upright posture. Circulation 78: 35-40.
- Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, et al. (1987) Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med 316: 1514-1518.
- Panza JA, Epstein SE, Quyyumi AA (1991) Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med 325: 986-990.
- Vilas D, Gomis M, Blanco M, Cortes J, Millan M, et al. (2012) Circadian rhythms in the efficacy of intravenous alteplase in patients with acute ischemic stroke and middle cerebral artery occlusion. ChronobiolInt 29: 1383-1389.
- Jain S, Namboodri KK, Kumari S, Prabhakar S (2004) Loss of circadian rhythm of blood pressure following acute stroke. BMC Neurol 4: 1.
- Castilla-Guerra L, Espino-Montoro A, Fernandez-Moreno MC, Lopez-Chozas JM (2009) Abnormal blood pressure circadian rhythm in acute ischaemic stroke: are lacunar strokes really different? Int J Stroke 4: 257-261.
- Naess H, Idicula T, Brogger J, Waje-Andreassen U, Thomassen L (2011) High proportion of lacunar strokes at night: the Bergen stroke study. J Stroke Cerebrovasc Dis 20: 424-428.
- Lip GY, Tan EK, Lau CK, Kamath S (2001) Diurnal variation in stroke onset in atrial fibrillation. Stroke 32: 1443-1448.
- Spengos K, Vemmos K, Tsivgoulis G, Manios E, Zakopoulos N, et al. (2003) Diurnal and seasonal variation of stroke incidence in patients with cardioembolic stroke due to atrial fibrillation. Neuroepidemiology 22: 204-210.
- Li-Saw-Hee FL, Blann AD, Lip GY (2000) A cross-sectional and diurnal study of thrombogenesis among patients with chronic atrial fibrillation. J Am CollCardiol 35: 1926-1931.
- Riccio PM, Klein FR, PaganiCassará F, Muñoz Giacomelli F, González Toledo ME, et al. (2013) Newly diagnosed atrial fibrillation linked to wake-up stroke and TIA: hypothetical implications. Neurology 80: 1834-1840.
- Takahashi JS, Hong HK, Ko CH, McDearmon EL (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9: 764-775.
- Yagita K, Tamanini F, Yasuda M, Hoeijmakers JH, van der Horst GT, et al. (2002) Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J 21: 1301-1314.
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, et al. (2008) Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 58: 78-88.
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, et al. (2002) The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251-260.
- Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, et al. (2007) Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. ProcNatlAcadSci U S A 104: 3342-3347.
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, et al. (2002) Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. CurrBiol 12: 540-550.
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, et al. (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78-83.
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, et al. (2002) A transcription factor response element for gene expression during circadian night. Nature 418: 534-539.
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, et al. (1999) Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284: 2177-2181.
- Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, et al. (2012) Human responses to bright light of different durations. J Physiol 590: 3103-3112.
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. ProcNatlAcadSci U S A 108: 1657-1662.
- Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, et al. (2008) Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J PhysiolRegulIntegr Comp Physiol 294: R1675-1683.
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. ProcNatlAcadSci U S A 106: 4453-4458.
- McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, et al. (2001) Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105: 877-889.
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, et al. (2000) Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682-685.
- Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929-937.
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, et al. (2004) Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119: 693-705.
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, et al. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. ProcNatlAcadSci U S A 101: 5339-5346.
- Silver R, LeSauter J, Tresco PA, Lehman MN (1996) A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382: 810-813.
- Prolo LM, Takahashi JS, Herzog ED (2005) Circadian rhythm generation and entrainment in astrocytes. J Neurosci 25: 404-408.
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, et al. (2002) Circadian rhythms in isolated brain regions. J Neurosci 22: 350-356.
- Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S (2006) The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. ProcNatlAcadSci U S A 103: 5591-5596.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, et al. (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034-2041.
- Cavalcanti P, Campos T, Araujo J (2012) Actigraphic analysis of the sleep-wake cycle and physical activity level in patients with stroke: implications for clinical practice. ChronobiolInt 29: 1267-1272.
- Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA (2009) Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med 35: 781-795.
- Tischkau SA, Cohen JA, Stark JT, Gross DR, Bottum KM (2007) Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. ExpNeurol 208: 314-322.
- Boone DR, Sell SL, Micci MA, Crookshanks JM, Parsley M, et al. (2012) Traumatic brain injury-induced dysregulation of the circadian clock. PLoS One 7: e46204.
- Sun Y, Yang Z, Niu Z, Wang W, Peng J, et al. (2006) The mortality of MOP3 deficient mice with a systemic functional failure. J Biomed Sci 13: 845-851.
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, et al. (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoSBiol 2: e377.
- Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, et al. (2010) CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. ProcNatlAcadSci U S A 107: 19090-19095.
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, et al. (2005) Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 41: 122-132.
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868-1873.
- Lefta M, Campbell KS, Feng HZ, Jin JP, Esser KA (2012) Development of dilated cardiomyopathy in Bmal1-deficient mice. Am J Physiol Heart CircPhysiol 303: H475-485.
- Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, et al. (2007) Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 115: 2188-2195.
- Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, et al. (2012) Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18: 774-782.
- Wiebking N, Maronde E, Rami A (2013) Increased neuronal injury in clock gene Per-1 deficient-mice after cerebral ischemia. CurrNeurovasc Res 10: 112-125.
- Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, et al. (2002) NPAS2: a gas-responsive transcription factor. Science 298: 2385-2387.
- Lukat-Rodgers GS, Correia C, Botuyan MV, Mer G, Rodgers KR (2010) Heme-based sensing by the mammalian circadian protein CLOCK. InorgChem 49: 6349-6365.
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, et al. (2007) Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318: 1786-1789.
- Li M, Gallo D, Csizmadia E, Otterbein LE, Wegiel B (2014) Carbon monoxide induces chromatin remodelling to facilitate endothelial cell migration. ThrombHaemost 111.
Citation: Schallner N, LeBlanc R, Otterbein LE, Hanafy KA (2014) Circadian Rhythm in Stroke – The Influence of Our Internal Cellular Clock on Cerebrovascular Events. J Clin Exp Pathol 4:163. Doi: 10.4172/2161-0681.1000163
Copyright: © 2014 Schallner N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 17842
- [From(publication date): 3-2014 - Apr 24, 2024]
- Breakdown by view type
- HTML page views: 13302
- PDF downloads: 4540