Epidemiologic, Clinicopathological, Immunohistochemical and Molecular Analysis of Gastrointestinal Glomus Tumors
Received: 18-Jul-2014 / Accepted Date: 08-Sep-2014 / Published Date: 11-Sep-2014 DOI: 10.4172/2161-0681.1000189
Abstract
Glomus tumors are uncommon lesions that may even occur in the gastrointestinal tract. We collected 6 cases of glomus tumors (5 females, 1 male) with a median age of 65.5 years (range 48-86 years) arising from stomach (5 cases) and colon. The tumor sizes varied from 1.5 to 3.5 cm (median 2.5 cm). In our Institutions, the frequency of gastrointestinal glomus tumor is 1 case/57 Gastrointestinal Stromal Tumors (GIST). Histologically and immunohistochemically gastrointestinal glomus tumors are very identical to the conventional soft tissue counterpart, expressing smooth-muscle actin and collagen IV, but not CD117, DOG1, neuroendocrine markers, S100, cytokeratins, desmin. A molecular analysis for c-KIT and PDGFRalpha and PDGFRbeta mutations was performed with negative results by direct sequencing.
Our data confirm that glomus tumors may rarely occur in gastrointestinal tract and differential diagnosis includes several epithelial and mesenchymal tumors, more commonly arising in this site. Histology is quite peculiar, but immunostains may be helpful in differential diagnosis. No mutations were detected in c-KIT and PDGFRalpha and PDGFRbeta.
Keywords: Glomus tumor; Stomach; GIST; Immunohistochemistry; c-KIT; PDGFR
Introduction
Glomus tumors are mesenchymal neoplasms composed of modified smooth muscle cells representing the neoplastic counterpart of the perivascular glomus bodies (the neuromyoarterial canal of Sucquet- Hoyer) [1]. These tumors usually occur in peripheral soft tissue, especially in the distal part of extremities [2]. In the gastrointestinal tract, glomus tumors are most commonly found in the antral region of the stomach [3-5].
They mainly occur in females in the sixth decade and at endoscopy appear as an intramural or submucosal nodule protruding into the visceral lumen [3-6].
The great majority are benign, but exceedingly rare cases of malignant glomus tumors are reported [7]. Glomus tumors in the gastrointestinal tract may be incidental, asymptomatic findings, but bleeding, epigastric discomfort and pain, nausea and vomiting are the most common symptoms [3-6]. Histologically, glomus tumor consists is highly-vascularised with endothelial cells surrounded by a mantle of small, uniform, rounded-to-oval cells, with central, round nucleus and amphophilic to lightly eosinophilic cytoplasm. Characteristically each cell is surrounded by basal lamina. The tumor cells are positive for smooth-muscle actin, h-caldesmon, calponin and collagen IV, but negative for CD117, CD34, desmin, S100, epithelial and neuroendocrine markers [6,7].
No molecular characteristics have been reported in this tumor and only a recent work analyzed the mutational set-up in c-KIT and PDGFRalpha genes with negative results. The purpose of this study is detail on the epidemiologic, clinicopathologic, immunohistochemical and molecular features of gastrointestinal glomus tumors consecutively collected from 2 different Institutions in a homogeneous Caucasian population.
Materials and Methods
After a pathological review of mesenchymal tumors of the gastrointestinal tract from January 2000 to June 2014 in 2 different Institutions (Operative Units of Pathologic Anatomy of the Azienda Policlinico of Modena and Azienda St. Maria Nuova of Reggio Emilia, Italy), we collected 6 cases of glomus tumors. In the same period of time, 324 gastrointestinal stomal tumors were detected.
Clinical information (age, sex, symptoms, tumor site, tumor size, computed-tomography imaging studies, therapy and follow up) were recorded from medical charts. A consensus diagnosis was reached after histologic review at multiheaded microscope with all pathologists involved in the study and according to the WHO criteria for the diagnosis of glomus tumor [8].
Immunohistochemistry
For each patient, 4-micron thick sections were obtained from a representative block. Sections were air dried overnight at 37°C and then deparaffinized in xylene and rehydrated through a decreasing concentration of alcohol to water. Endogenous peroxidase activity was blocked by immersion for 10 minutes with 3% hydrogen peroxide (H2O2) in methanol. Incubation with primary antibodies was accomplished with a modified streptavidin-biotinperoxidase technique using an automated immunostainer (Benchmark, Ventana, Strasbourg, France); 3’-3-diaminobenzidine was used as the chromogene, and Harris’s hematoxylin was used as the counterstain. The antibodies used in the study and their technical characteristics are listed in Table 1. Negative and positive controls were included in each batch.
| Primer Name | Primer sequence | Annealing temperature | Product Size (bp) |
|---|---|---|---|
| Exon 9 c-KIT | Forward5’- ATG CTC TGC TTC TGT ACT GCC-3’ Reverse 5’- CAG AGC CTA AAC ATC CCC TTA –3’ |
58°C | 238 bp |
| Exon 11 c-KIT | Forward 5’- GAT CTA TTT TTC CCT TTC TC-3’ Reverse 5’- AGC CCC TGT TTC ATA CTG AC –3’ |
57°C | 174 bp |
| Exon 13 c-KIT | Forward 5’- GCT TGA CAT CAG TTT GCC AG-3’ Reverse 5’- AAA GGC AGC TTG GAC ACG GCT TTA –3’ |
66°C | 193 bp |
| Exon 14 c-KIT | Forward 5’- GTC TGA TCC ACT GAA GCT G-3’ Reverse 5’- ACC CCA TGA ACT GCC TGT C –3’ |
59°C | 151 bp |
| Exon 17 c-KIT | Forward 5’- TAC AAG TTA AAA TGA ATT TAA ATG GT-3’ Reverse 5’- AAG TTG AAA CTA AAA ATC CTT TGC –3’ |
63°C | 228 bp |
| Exon 12 PDGFRa | Forward 5’-TCC AGT CAC TGT GCT GCT TC-3’ Reverse 5’-GCA AGG GAA AAG GGA GTC TT-3’ |
56°C | 260 bp |
| Exon 14 PDGFRa | Forward 5’-GTA GCT CAG CTG GAC TGA TA-3’ Reverse 5’-AAT CCT CAC TCC AGG TCA GT-3’ |
55°C | 180 bp |
| Exon 18 PDGFRa | Forward 5’-TAC AGA TGG CTT GAT CCT GAG T-3’ Reverse 5’-AGT GTG GGA GGA TGA GCC TG-3’ |
59°C | 211 bp |
| Exon 12 PDGFRB | Forward 5’-TAA TTC CTG GGG TTG GTC CTC-3’ Reverse 5’-AAC TTG AGT CCC CAC ACT GCC-3’ |
52°C | 174 bp |
| Exon 14 PDGFRB | Forward 5’-GGG GCA GAA GAG TCA GAA TAG-3’ Reverse 5’-GGA GTG TGC TGT TGT GCA AG-3’ |
63°C | 300 bp |
| Exon 18 PDGFRB | Forward 5’-CCC AAA GCC CTT GAC ATG AAG-3’ Reverse 5’-ACT GGT CAG GAG GGA ATC TG-3’ |
56°C | 274 bp |
Table 1: Oligonucleotide primers used for sequence analysis of the indicated genes.
For each antibody, the percentage of positive cells and the intensity of staining (0, negative; 1+, weak; 2+, moderate; and 3+, strong) were recorded. A tumor was considered positive for markers if more than 10% of the neoplastic cells reacted with an intensity of 2+ or greater on the relevant subcellular localization. All the primary antibodies used in the study are prediluted and purchased from Ventana. The clones are the following: smooth-muscle actin (clone 1A4), chromogranin A (clone LK2H10), CD117 (clone A4502), CD34 (QBEnd/10), Collagen IV (clone CIV22), cytokeratins (clone AE1/AE3), DOG1 (clone SP31), desmin (clone DE-R-11), S100 (clone 4C4.9), synaptophysin (SP11), Ki67 (clone MIB-1).
Molecular Biology
Several 5-micron thick sections obtained from a representative paraffin-embedded block were deparaffinized by xylene, and tumor DNA from microdissected cells was subject to proteinase K treatment in an extraction buffer (50 mmol/L Tris-HCl, pH 8.0; 1 mmol/L EDTA; and 0.5% Tween-20) and then incubated overnight at 37°C. Polymerase chain reaction (PCR) was performed in 10 _L reactions containing 1.0 _L DNA, 10 mmol/L Tris-HCl (pH 8.3), 40 mmol/L KCl, 1.0 to 1.5 mmol/L MgCl2, 200 mmol/L dNTP, 20 pM of each primer, and 0.25 U Platinum Taq polymerase (Invitrogen, Carlsbad, CA). PCR reaction was carried out on Uno II Thermoblock.
Biometra, Gottingen, Germany: Initial denaturation at 94°C for 3 minutes was followed by 41 cycles and a final extension step (5 minutes at 72°C). The cycles included denaturation at 95°C for 1 minute, annealing at 55 to 58°C for 1 minute, and extension at 72°C for 2 minutes. The amplified DNA was electrophoresed on 1% lowmelt agarose gel for 1 hour. The amplification products were then excised from the gel and purified by using Wizard PCR Preps-DNA Purification System (Promegar Corp, Madison WI) as indicated by the manufacturer’s instructions. PCR products were then sequenced in both directions with BigDye Terminator (Applied Biosystems, Weiterstadt, Germany) sequencing kit using the same primers as used for PCR. PCR products were finally purified by Centri-Sep Spin Columns and subsequently analyzed using the ABI Prism 310 sequence analyzer (Applied Biosystems). The forward and reverse oligonucleotide primers used to amplify c-KIT exons 9, 11, 13, 14 and 17; PDGFRalpha exons 12, 14 and 18, and PDGFRbeta exons 12, 14, and 18 are listed in Table 1.
Results
The main clinicopathologic characteristics are summarized in Table 2. From an epidemiologic view, the ratio between glomus tumor and gastrointestina stromal tumor in 1/57. There is a marked prevalence in females (5 cases) and the mean age of the patients is 65.5 years (range 48-86 years). Four patients were symptomatic, complaining of hematemesis, discomfort, nausea and melaena. The lesions were incidental findings in 2 cases. The tumor was located in the stomach in 5 cases (4 on the antrum, 1 on the body) and in the right colon in 1 case. All patients received complete, margin-free, surgical resection of the tumor without adjuvant therapy and are alive and well at a mean follow-up of 68 months (range 8-156 months).
| Age/Gender | Symptoms | Site | Size/Mitoses# | Imaging | Therapy | Survival |
|---|---|---|---|---|---|---|
| 86/M | Incidental | S | 1.5/- | Nodule | Surgery | AW |
| 51/F | Hematemesis | S | 3.5/1 | Nodule | Surgery | AW |
| 63/F | Epigastricdiscomfort | S | 1-Mar | Nodule | Surgery | AW |
| 79/F | Nausea | S | 2/- | Nodule | Surgery | AW |
| 48/F | Melaena | C | 1-Mar | Nodule | Surgery | AW |
| 67/F | Incidental | S | 2/- | Nodule | Surgery | AW |
Table 2: Clinico-pathologic and radiologic characteristics of glomus tumors.
In all cases, the endoscopic examination revealed a submucosal masses protruding into the lumen and sparing the overlying mucosa. The tumor size ranged from 1.5 to 3.5 cm (median size, 2.5 cm). None of the cases was correctly diagnosed on the clinical and endoscopic ground and GIST, leiomyoma, lipoma and neuroendocrine tumor were the initial considerations.
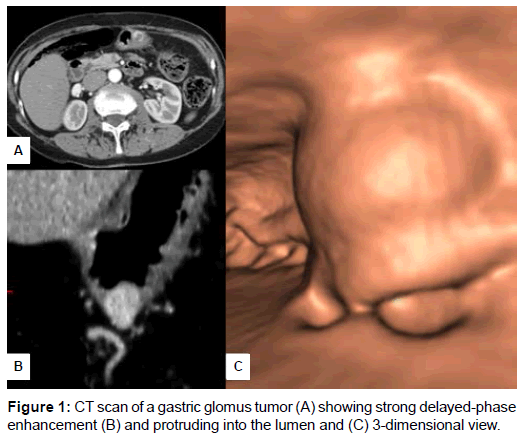
In gastric cases, the endoscopic ultrasonography showed an inhomogeneous, hypoechoic round mass in the submucosal layer. Data from CT scan were available in 4 out of 6 cases. All CT imaging studies refer to gastric glomus tumor and evidenced a rounded nodule with well-defined, smooth borders of the gastric wall protruding into the visceral lumen. The lesions had strong and prolonged enhancement (Figure 1 A-C). The gross appearance on resected specimen showed relatively well-circumscribed, intraparietal nodules with whitish cut surface, rubbery consistency and haemorrhagic areas.
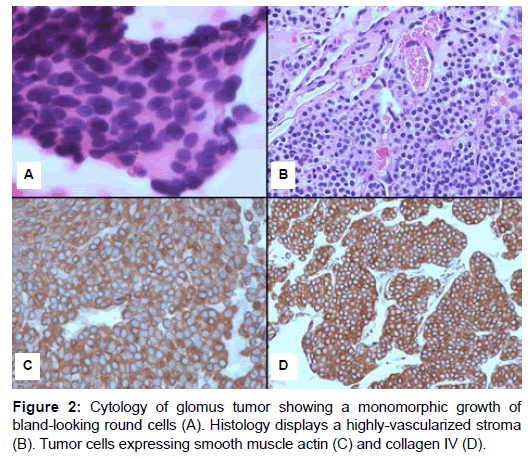
In one case the cytologic aspirate was performed and revealed tightly packed well-demarcated nests of round to poligonal, monomorphic epithelioid cells lacking significant atypia (Figure 2A). The cell borders were ill-defined. The nuclei were round to oval, centrally located with a granular chromatin pattern and inconspicuous nucleoli. The background of the smears contained blood, occasional inflammatory cells and was devoid of necrosis. Microscopic examination of histologic slides revealed a very distinctive morphology with a tumor composed of solid nests of small, uniform, rounded-to-oval cells, with central, round nucleus, inconspicuous nucleoli and amphophilic to lightly eosinophilic cytoplasm with ill-defined, marked cell borders (Figure 2B). These cells were in close proximity to numerous small blood vessels and tumor nodules were separated by hyalinized collenous stroma. Large, dilated and thick-walled vascular spaces with some clots were noted. Mitotic figures per 10 high-power-fields ranged from none to 1. Necrosis was absent.
At immunohistochemistry (Table 3), all cases tumors were positive for Smooth-muscle Actin (SMA) and collagen type IV with a pericellular net-like positivity (Figure 2C and D). All the other tested markers were completely negative, while weak expression with synaptophysin was noted in 1 case. All cases were wild-type for mutational analysis of c-KIT, PDGFRalpha and PDGFRbeta genes.
| Case | SMA | DES | S100 | ChrA | Syn | CD117 | DOG1 | CKs | CD34 | ColIV | Molecular analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | + | - | - | - | - | - | - | - | - | + | wild-type |
| #2 | + | - | - | - | - | - | - | - | - | + | wild-type |
| #3 | + | - | - | - | -/+ | - | - | - | - | + | wild-type |
| #4 | + | - | - | - | - | - | - | - | - | + | wild-type |
| #5 | + | - | - | - | - | - | - | - | - | + | wild-type |
| #6 | + | - | - | - | - | - | - | - | - | + | wild-type |
Table 3: Immunohistochemical and molecular characteristics of Glomus Tumors.
Discussion
Glomus tumor of the stomach was first described by De Busscher in 1948 as a rare, usually solitary lesion that is found as an intramural nodule [9]. We observed here that the frequency of glomus tumors is about 1 case every 57 GISTs, a figure significantly lower than that recently reported by Wang et al. [10] accounting for a ratio of 1/145 GISTs. However, these discrepant results may be related to several factors, particularly the specialization of the referring centre and possibly the different ethnicity of the analyzed population. According to the literature, glomus tumor observed in our case series mainly occur in female gender and sixth decade of life [3-6,10]. All but one involved the stomach which represents the most common gastrointestinal site.
Clinical manifestations (epigastric discomfort, bleeding, nausea) are consistent with previous works and in 2 cases the tumor was incidentally detected in an asymptomatic patient. All tumors in our series were solitary, with a mean size of 2.5 cm and up to 1 mitosis per 10 high-power field. Since, deep location, tumors larger than 5 cm and mitotic rate higher than 2 mitoses per 10 high-power field are considered to have some potential of malignancy, [7] all cases here included should be considered as benign lesions. In fact, none of our patients experienced recurrences/metastasis and are all alive and well at a mean follow-up of 68 months.
Several lesions can arise in the stomach as submucosal masses that project into the lumen or out onto the serosa and differential diagnosis is challenging on clinical and imaging ground, [3,4,10,11]. In particular, gastrointestinal glomus tumors must be differentiated from GIST, neuroendocrine tumor, paraganglioma, lymphoma, leiomyoma, schwannoma, granular cell tumor, metastatic melanoma and clear cell sarcoma. Immunohistochemical and molecular helpful features in differential diagnosis are reported in Table 4.
| Lesion | SMA | Coll IV | DES | CD117 | CD34 | NEm | S100 | LCA | CKs | Molecular Testing |
|---|---|---|---|---|---|---|---|---|---|---|
| GlomusTumor | + | + | - | - | - | - | - | - | - | none |
| GIST | -/+* | -/+ | - | + | +/- | - | - | - | - | c-KIT, PDGFRa, BRAF, SDH |
| Carcinoid | - | - | - | - | - | + | - | - | + | none |
| Paraganglioma | - | - | - | - | + | +^ | - | - | ||
| Leiomyoma | + | - | + | - | - | - | - | - | - | none |
| Schwannoma | - | - | - | - | - | - | +§ | - | - | none |
| Granular cell tumor | - | - | - | - | - | - | +§ | - | - | none |
| Melanoma | - | - | - | -/+ | - | - | +§ | - | - | BRAF, c-KIT |
| Lymphoma | - | - | - | - | - | - | - | + | - | none |
Table 4: Helpful findings in differential diagnosis between Glomus Tumors and other mimicking lesions.
Among these lesions gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the digestive tract [11,12]. GIST are distinct spindle, epithelioid or mixed mesenchymal tumors typically defined by immunohistochemical expression of KIT (CD117). Many GISTs have KIT-activating mutations of exon 11 or exon 9. Approximately 10-15% of GISTs lacking KIT mutation harbour a mutation in PDGFRA, BRAF or SDH complex, while approximately 10% of GISTs are CD117-negative and DOG1 appears more sensitive than CD117 in these difficult cases [13]. The epithelioid GISTs can have a nested pattern, sometimes arranged around prominent dilated vascular spaces, giving them a resemblance to glomus tumor. Immunohistochemical differences between glomus tumor and epithelioid GIST are substantial. By contrast with GISTs, glomus tumor are consistently negative for CD117, DOG1 and CD34.
Neuroendocrine tumors are composed of uniform round cells, and a coarser chromatin pattern similarly to glomus tumor, but cell border are not as prominent as seen in glomus tumor. Neuroendocrine cells are strongly positive for cytokeratins, chromogranin A and synaptophisyn, but negative with SMA and collagen IV. Some variants of paraganglioma can closely mimic a solid variant of glomus tumor. However, paraganglioma is strongly positive for chromogranin A and synaptophisin and typically have S100-positive sustentacular cells that are absent in glomus tumor [8].
Malignant lymphoma, such as gastric MALT-type marginal-zone lymphoma, may histologically resemble a glomus tumor with a diffuse pattern, particularly on small biopsy or cytology. Lymphoid markers are very helpful in differential diagnosis. A case of gastric glomus tumor containing foci of mantle cell lymphoma has been reported [14]. Leiomyomas are composed of cigar-shaped smooth muscle cells with eosinophilic cytoplasm with bland, uniform, cigar-shaped nuclei, expressing SMA and desmin-positive arranged in intersecting fascicles [15].
Gastrointestinal schwannomas look like an expansive, nonencapsulated, well-circumscribed mass with an outer lymphoid-cuff. They are composed of bundles of spindle cells mixed with wavy collagen fibers. All schwannomas in the digestive tract are S100-positive and negative for CD117, CD34, SMA, desmin, collagen IV and cytokeratins [16,17].
Granular cell tumors are composed of sheets of oval-to-polygonal cells with a small central nucleus and abundant granular slightly basophilic cytoplasm. Granular cell tumors are typically positive only with S100 protein [18]. Metastatic amelanotic melanoma and clear cell sarcoma can involve the gastrointestinal tract. These tumors may show a variety of histologic patterns, including epithelioid, spindled, round and pleomorphic cellular patterns, some of these simulating glomus tumor. Positivity for S100 protein and other melanoma markers rules out glomus tumor [8,19]. Among all markers, collagen type IV revealed the same sensitivity of SMA (expressed in smooth-muscle tumors and a subset of GISTs from the small intestine), but it is superior in specificity since only a minor subset of GISTs may show a weak-to-moderate expression with collagen IV (unpublished personal observation).
Since fine-needle aspiration and small biopsies offer a rapid method in the diagnosis of gastrointestinal tumors, pathologists should consider that cytological features of glomus tumor may be quite specific showing tightly packed clusters of relatively uniform, small, round to polygonal cells, with areas lined by endothelial cells, and a clean background with haemorrhagic features [20].
Only a few experiences have investigated molecular characteristics of glomus tumor. According to the previous works by Miettinen et al [3] and more recently by Wang et al, [10] no mutational events have been identified in c-KIT and PDGFRalpha. Interestingly, we first evidenced no mutations also in PDGFRbeta gene.
In summary, glomus tumor may occasionally involve the gastrointestinal tract and have the same histological and immunophe notypical features of the more conventional soft tissues counterpart. In the digestive tract, glomus tumors mainly affect females at the fifth/sixth decade of life and the gastric site. Differential diagnosis is almost impossible on imaging studies and diagnosis depends on the pathologist skills [21,22]. A small panel of immunostains are very helpful in discriminating glomus tumor from other mimicking lesions. Conservative, margin-free, resection is curative. Gastrointestinal glomus tumors do not harbor gene alterations in c-KIT, PDGFRalpha and PDGFRbeta.
References
- Tsuneyoshi M, Enjoji M (1982) Glomus tumor: a clinicopathologic and electron microscopic study. Cancer 50: 1601-1607.
- Einzinger FM, Weiss SW (2008) Perivascular tumors. In: Weiss SW, Goldblum JR editors. Soft tissue tumors (5th Edn) St. Louis: C.V. Mosby Co.: 751-65
- Miettinen M, Paal E, Lasota J, Sobin LH (2002) Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J SurgPathol 26: 301-311.
- Kang G, Park HJ, Kim JY, Choi D, Min BH, et al. (2012) Glomus tumor of the stomach: a clinicopathologic analysis of 10 cases and review of the literature. Gut Liver 6: 52-57.
- Lee HW, Lee JJ, Yang DH, Lee BH (2006) Aclinicopathologic study of glomus tumor of the stomach. J ClinGastroenterol 40: 717-720.
- Wang LM, Chetty R (2012) Selected unusual tumors of the stomach: a review. Int J SurgPathol 20: 5-14.
- Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW (2001) Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J SurgPathol 25: 1-12.
- Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO Classification of Tumors of the Digestive System (IVthEdn) IARC Lyon.
- DE BUSSCHER G (1948) [Not Available]. ActaNeerlMorphol Norm Pathol 6: 87-105.
- Wang ZB, Yuan J, Shi HY (2014) Features of gastric glomus tumor: a clinicopathologic, immunohistochemical and molecular retrospective study. Int J ClinExpPathol 7: 1438-1448.
- Dow N, Giblen G, Sobin LH, Miettinen M (2006) Gastrointestinal stromal tumors: differential diagnosis. SeminDiagnPathol 23: 111-119.
- Miettinen M, Lasota J (2001) Gastrointestinal stromal tumors: definition, clinical, histological, immunoistochemical and molecular genetic features and differential diagnosis. Virchows Arch; 438: 1-12
- Olga O, Greenson JK (2014) New issues in gastrointestinal stromal tumors of the stomach. DiagnHistopathol 20: 222-227
- West AB, Buckley PJ (1992) Mantle zone lymphoma in a gastric glomus tumor. Cancer 70: 2246-2249.
- Lee MJ, Lim JS, Kwon JE, Kim H, Hyung WJ, et al. (2007) Gastric true leiomyoma: computed tomographic findings and pathological correlation. J Comput Assist Tomogr 31: 204-208.
- Sarlomo-Rikala M, Miettinen M (1995) Gastric schwannoma--a clinicopathological analysis of six cases. Histopathology 27: 355-360.
- Chetty R (2011) Reticular and microcysticschwannoma: a distinctive tumor of the gastrointestinal tract. Ann DiagnPathol 15: 198-201.
- White JG, el-Newihi HM, Hauser CJ (1994) Granular cell tumor of the stomach presenting as gastric outlet obstruction. Am J Gastroenterol 89: 2259-2260.
- Pauwels P, Debiec-Rychter M, Sciot R, Vlasveld T, den Butter B, et al. (2002) Clear cell sarcoma of the stomach. Histopathology 41: 526-530.
- Vinette-Leduc D, Yazdi HM (2001) Fine-needle aspiration biopsy of a glomus tumor of the stomach. DiagnCytopathol 24: 340-342.
- Gu M, Nguyen PT, Cao S, Lin F (2002) Diagnosis of gastric glomus tumor by endoscopic ultrasound-guided fine needle aspiration biopsy. A case report with cytologic, histologic and immunohistochemical studies. ActaCytol 46: 560-566.
- Debol SM, Stanley MW, Mallery S, Sawinski E, Bardales RH (2003) Glomus tumor of the stomach: cytologic diagnosis by endoscopic ultrasound-guided fine-needle aspiration. DiagnCytopathol 28: 316-321.
Citation: Mengoli MC, Nosseir S, Mataca E, Bordi P, Lupi M, et al. (2014) Epidemiologic, Clinicopathological, Immunohistochemical and Molecular Analysis of Gastrointestinal Glomus Tumors. J Clin Exp Pathol 4:189. Doi: 10.4172/2161-0681.1000189
Copyright: © 2014 Mengoli MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14786
- [From(publication date): 11-2014 - Apr 19, 2024]
- Breakdown by view type
- HTML page views: 10427
- PDF downloads: 4359