Epidemiology of Cryptococcal Meningitis Associated with HIV in an Indian Hospital
Received: 25-Jun-2014 / Accepted Date: 25-Jul-2014 / Published Date: 01-Aug-2014 DOI: 10.4172/2161-1165.1000166
Abstract
Cryptococcus neoformans is an emerging pathogen especially in the setting of HIV infected patients with meningitis. The infection usually manifests in patients with impaired cell mediated immunity. Forty six culture positive cases of cryptococcal meningitis were identified over a period of three years from a tertiary care institute in Delhi. The patients were predominantly males (91%) of reproductive age group, mostly residing in and around Delhi. Most significant co-infections were HIV infection in 93% and tuberculosis in 43.5% cases. All forty six strains belonged to Cryptococcus neoformans var grubii, molecular type VN I, mating type α (MAT α). There was 100% susceptibility to amphotericin B and azoles, one isolate was resistant to 5-fluorocytosine (MIC 64 μg/ml). Cerebrospinal fluid in symptomatic HIV patients should be carefully screened for Cryptococcus spp. which should be identified and also typed for epidemiological purposes.
Keywords: Cryptococcosis; Meningoencephalitis; HIV
Introduction
Cryptococcosis is a globally prevalent mycosis and a major cause of fungal meningoencephalitis in immunocompromised patients. According to WHO estimates, the disease is most common in sub-saharan Africa and the annual incidence of cryptococcal meningitis is about one million worldwide, resulting in 625,000 deaths, most of these cases among people with HIV/AIDS [1]. The incidence of cryptococcal meningitis ranges from 0.04 to 12% per year among persons with HIV, approximately two thirds die within 3 months of infection [1]. Early diagnosis and initiation of appropriate antifungal therapy may reduce mortality in these patients. The objective of the study was to retrospectively analyse the risk factors, co-morbid conditions and clinical outcome in patients with culture positive Cryptococcus isolates. Speciation, identification of the mating and molecular types and antifungal susceptibility patterns of C. neoformans species complex from these patients of were performed.
Material and Methods
This was a retrospective observational study in which the medical records of patients of cryptococcal meningitis were analyzed in view of their demographics, presenting complaints, HIV status, co -morbid conditions, diagnostic tests, treatment and outcome. Suspected CSF specimens were subjected to direct India ink examination, urease test and culture. Presumptive identification as C. neoformans species complex was done by salient physiological features manifested by the colonies growing on Saboraud Dextrose agar (SDA) at 37ºC, positive urease test and chocolate brown coloured yeast-like colonies on niger seed agar medium. They were confirmed by ID 32 C carbon assimilation profiles, read and interpreted by the mini API system (Biomerieux). In vitro antifungal susceptibility of all confirmed isolates was determined by broth microdilution method [2] for amphotericin B (AMB), 5-fluorocytosine (5FC), fluconazole, ketoconazole, voriconazole and itraconazole. The mating types of strains were determined as described by Yan et al. [3]. The strains were genotyped by PCR fingerprinting with the repetitive oligonucleotide (GACA) 4 used as a single primer.
Results
A total of 1172 cerebrospinal fluid cultures were received over a period of three years of which forty six were diagnosed as cases of Cryptococcus neoformans meningitis. All these patients were adults aged 18-53 years, 42 were males and 4 females. Most of these were residents of Delhi (65.2%); others were from adjoining states of Haryana and Uttar Pradesh.
The most common presenting symptoms were headache (69.5%) and altered sensorium (43.4%). Standard treatment guidelines were followed [4] which included Amphotericin B deoxycholate (AmBd); 0.7–1.0 mg/kg/day intravenously plus flucytosine (100 mg/kg/day) orally in 4 divided doses for 2 weeks, followed by fluconazole (6 mg/kg/day orally) for a minimum of 8 weeks. Thirty patients were treated in this manner. Of these, eighteen (60%) patients showed CSF clearance and recovered, however, one (5.5%) patient relapsed after one month of completed treatment. Twelve patients (40%) died while undergoing treatment in the hospital; six during first week of treatment, three each during the second and third weeks of treatment. Cause of death in these patients was attributed to renal impairment, jaundice, or associated infections like tuberculosis. Two patients developed renal failure within the first week of initiating treatment, therefore managed conservatively. Treatment could not be given to five terminally ill patients due to associated renal failure (2), aspiration pneumonia (2), hepatitis following introduction of antitubercular therapy (1). Treatment details for remaining patients were not available. A summary of patient characteristics and treatment has been tabulated in Table 1.
| Characteristics of Patients of Cryptococcal meningitis | Total no. (%) |
|---|---|
| Sex Males Females |
42 (91.3%) 04 (8.7%) |
| Marital Status Married Unmarried |
36 (78.2%) 10 (21.8%) |
| Occupation Government setting Private setting Driver Farmer |
18 (39.13%) 14 (30.4%) 7 (15.2%) 7 (15.2%) |
| Patients treated according to Standard guidelines Complete treatment and recovery Mortality during treatment |
30 (65.2%) 18 12 |
| Patients not treated according to standard guidelines Renal failure Aspiration pneumonia Drug induced hepatitis Unknown Causes |
16 (34.8%) 04 02 01 09 |
Table 1: Characteristics and Treatment Summary of Patients with Cryptococcal meningitis
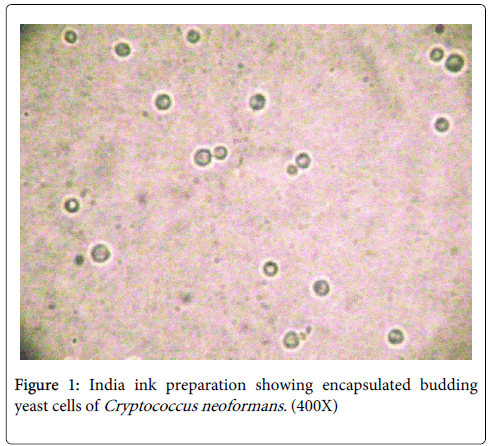
Encapsulated budding yeast cells (Figure 1) were seen in forty five specimens on India ink preparation (98%). Cryptococcus neoformans grew in routine fungal culture media, most often within two days of incubation, brown pigmented colonies appeared on niger seed agar within four days at 30°C and urease test was positive. All strains were negative in canavanine-glycine-bromthymol blue (CGB) test, hence identified as Cryptococcus neoformans var grubii. All the isolates were susceptible to amphotericin B, fluconazole, ketoconazole, voriconazole and itraconazole, except one (2.1%) which was resistant to 5-fluorocytosine. Mating type PCR results showed that all isolates were mating type a (MAT a) and PCR fingerprinting analyses identified all forty six strains as C. neoformans var grubii, molecular type VNI. Details of genotyping of these isolates have been published in a study from India by Chowdhary et al. [5].
HIV antibody testing was done in forty three patients of whom forty showed positive results (93%). Of the three HIV negative patients, one patient expired (Table 2). Co-morbid condition in his case was diabetic nephropathy, chronic obstructive pulmonary disease and hemiparesis due to a cerebrovascular accident. Of the other two patients, one was a farmer with disseminated tuberculosis and other was a 42 year old male shopkeeper with no known risk factors. In this patient, India ink result was negative, urease test positivity within four hours was the first indication of cryptococcal meningitis. CD4 T-lymphocyte counts of HIV patients were determined using FACS Count flow cytometer/ FACS Calibur (Becton Dickinson, USA) and varied from 09 to 170 per cu mm, mean being 46 per cu mm. Co-existing tuberculosis was found in 20 (43.5%) patients including four extra-pulmonary cases; oral/ esophageal candidiasis in 06 (13%) patients, gastrointestinal symptoms including diarrhea/ dysentery/ jaundice in four (8.7%); and otitis media, Rhodotorula fungemia, syphilis, Pneumocystis jiroveci pneumonia in one patient each.
| S. No | Age/ Sex | Residence | Marital status | Occupation | Treatment | Presenting Complaints | Provisional Diagnosis | Co- morbid conditions | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 42/M | New Delhi | Married | Shopkeeper | ATT, Amp B, Fluconazole, Quinine | Fever with headache | Tubercular meningitis | None | Recovered and Discharged |
| 2. | 40/M | Uttar Pradesh | Married | Farmer | Amp B and Fluconazole for 18 days, fluconazole on discharge | Fever with headache, visual disturbances | Chronic meningitis with bilateral exposure keratitis | Disseminated Koch's | Discharged after 6 weeks |
| 3. | 53/M | New Delhi | Married | Business | Ciprofloxacin | Headache, generalized weakness, irritability | Chronic meningitis | Cerebro-vascular accident with hemiparesis, Type II Diabetes mellitus with DKA, Nephropathy with COPD. | Expired after 6 days of admission |
Table 2: Summary of HIV negative cases.
Discussion
C. neoformans is ubiquitous in nature, yet the incidence of cryptococcosis is relatively low. Cryptococcus survives environmentally in the sexual form. Basidiospores which are 1-3µin size may aerosolize and deposit in host alveoli [6]. Infection in the host is kept under control by active cell mediated immunity but when immunity wanes as in HIV infection, virulence factors of Cryptococci- the polysaccharide capsule, phenoloxidases act to produce disease and spread it hematogenously to extrapulmonary tissues. They usually settle in brain to cause meningoencephalitis [7] which maybe fatal if left untreated [8]. Till early 1980s, cryptococcosis was a rare infection and as many as a third of all patients gave history of steroid use [9]. The numbers steadily increased with the advent of HIV pandemic, it is the third most common cause of central nervous system infection [10]. Up to 5-10% of HIV patients show evidence of cryptococcosis in late stages [11] and it may be the first manifestation in 26-45% of these patients [12]. HIV infection accounts for more than 80% of the predisposing factors [13]. In our study, 93% were HIV reactive. Predisposing factors among HIV negative individuals include glucocorticosteroid therapy, solid-organ transplantation, chronic organ failure (renal or hepatic) syndromes, rheumatologic disorders, chronic lung disease, hematologic or other malignancies, splenectomy, male sex, age>60 years [14]. However in one of our HIV negative patients, no risk factors were found. High dose of exposure continuously over prolonged periods could have caused the disease to manifest. The most common co-infections in our study were tuberculosis and oral/esophageal candidiasis, comparable to results in a South African study where tuberculosis (32%) and oral thrush (46%) were most common [15]. Co-infections may also delay therapeutic response and interfere with successful outcomes. HIV infected patients are also susceptible to other mycoses associated with impaired CMI, such as mucosal candidiasis, histoplasmosis, etc. in contrast to mycoses for which neutrophil is the crucial host defense, like systemic candidiasis, mucormycosis and aspergillosis during the initial HIV infection [16].
Laboratory diagnosis of C. neoformans meningitis can be established by direct India ink examination, fungal culture, immunodiagnosis or molecular methods. The sensitivity of India ink varies from 70–90% in HIV positive patients [17] and around 50% in HIV negative patients [14]. Culture on Sabouraud’s dextrose agar is confirmatory and allows antifungal susceptibility of the isolates. The sensitivity of Cryptococcal antigen detection ranges from 83% to 97% and the specificity from 93% to 100% [18]. Antibodies to C. neoformans are present in most of the healthy individuals; therefore antibody detection methods cannot be relied upon for diagnosis [19]. CD4 counts were very low in our study, which also indicate compromised CMI.
C. neoformans related mortality ranges from 10-12% in the developed nations to 50-70% in sub–Saharan Africa [20]. Even after optimal treatment mortality in HIV-associated cryptococcal meningitis has been reported to range from 10 to 25% [21]. Treatment against cryptococcosis should be initiated at the earliest as it is a potentially life threatening illness. Antifungal resistance is rare in C. neoformans yet susceptibility testing is recommended. Fluconazole resistance is most often associated with AIDS patients receiving azole maintenance therapy [22]. Resistance of C. neoformans to flucytosine has been reported to be low (< 2%) [23], but has been correlated with relapses in up to 30-40% of flucytosine monotherapy cases [8]. Cross-resistance among antifungal azoles and polyenes occurs at a frequency of 10-8 in C. neoformans and a single mutation might cause resistance to both these classes [24]. Therefore, combination therapy should be used to take advantage of different pharmacokinetics and synergistic mechanisms of these drugs. A significant increase in the MIC (> 4-fold) of the antifungal between the primary and subsequent isolates, might suggest treatment failure due to drug resistance, warranting a new therapeutic drug regimen [8]. In the present study, a single isolate was resistant to 5FC. Relapses even after effective therapy has been recorded in HIV patients where prostate may act as an effective reservoir [8]. One relapse was also noted in an HIV positive male during this analysis. In our study, 40% mortality was observed in patients undergoing treatment, mostly during the first two weeks when amphotericin B and flucytosine were administered. In a study done in Kenya on Nephrotoxicity of amphotericin B in the treatment of cryptococcal meningitis in acquired immunodeficiency syndrome patients, mortality was 30.5% during the initial two week follow-up period and around 90% of the patients showed deranged renal function tests [25].
Cryptococcus neoformans has been classified into two varieties: C. neoformans var neoformans, serotype D and C. neoformans var grubii, serotype A [7]. The most common variety, C. neoformans var. grubii, accounts for >90% of cryptococcal infections and is considered more pathogenic than strains of serotype D [11]. C. gattii can cause meningitis in the immunocompetent and show lesser response to antifungals, resulting in prolonged treatment and poor outcomes [26]. Cryptococcal strains have been divided into eight major genotypes by PCR fingerprint patterns: VNI and VNII (serotype A; var grubii), VNIII (hybrid serotype AD; var neoformans), VNIV (serotype D; var neoformans), and VGI to VGIV for serotypes B and C; var gattii [27]. VNI and VNII are distributed globally, VNI is more common [28] The C. neoformans a-mating type MATa (Aa) has been found to be significantly more virulent than the a-mating strain Aa [29]. In the present study all forty six isolates were Cryptococcus neoformans var. grubii molecular type VNI, mating type a (MAT a).
Conclusion
High index of suspicion is required to diagnose cryptococcal meningitis. The causative agent identified in patients presenting to our institute was C. neoformans var grubii and in majority, HIV was an associated risk factor. Primary resistance to antifungals was rare and only one relapse was recorded, therefore treatment with Amphotericin B must not be delayed if the patient can tolerate this drug. In conformity with the global data these isolates belonged to mating type MAT a and molecular type VNI. Direct urease test from CSF is recommended as it may help in early diagnosis of India ink negative cases. For improved outcomes, every attempt should be made to improve the host immunity during treatment.
Acknowledgements
Dr H.S. Randhawa who acknowledges the award of an Honorary Scientist position to the Indian National Science Academy, New Delhi for his expert guidance and Dr Anuradha Chowdhary, Head of Department, V.P. Chest Institute, Delhi for helping in molecular identification and susceptibility testing of these isolates.
References
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525-530.
- Hamza OJ, van den Bout-van den Beukel CJ, Matee MI, Moshi MJ, Mikx FH, et al. (2006) Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J Ethnopharmacol 108: 124-132.
- Yan Z, Li X, Xu J (2002) Geographic distribution of mating type alleles of Cryptococcus neoformans in four areas of the United States. J ClinMicrobiol 40: 965-972.
- Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, et al. (2000) Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis 30: 710-718.
- Chowdhary A, Randhawa HS, Sundar G, Kathuria S, Prakash A, et al. (2011) In vitro antifungal susceptibility profiles and genotypes of 308 clinical and environmental isolates of Cryptococcus neoformans var. grubii and Cryptococcus gattii serotype B from north-western India. J Med Microbiol60: 961-967.
- Ellis D, Pfeiffer T (1992) The ecology of Cryptococcus neoformans. Eur J Epidemiol 8: 321-325.
- Kwon-Chung KJ. Cryptococcosis. In: Kwon-Chung KJ, Bennett JE, eds. Medical Mycology. Philadelphia: Lea &Febiger, 1992:397-446.
- Mitchell TG, Perfect JR (1995) Cryptococcosis in the era of AIDS--100 years after the discovery of Cryptococcus neoformans. ClinMicrobiol Rev 8: 515-548.
- Lewis JL, Rabinovich S (1972) The wide spectrum of cryptococcal infections. Am J Med 53: 315-322.
- Levy RM, Bredesen DE, Rosenblum ML (1985) Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): Experience at UCSF and review of literature. J Neurosurg 62: 475-495.
- Casadevall A, Perfect JR (1998) Cryptococcus neoformans. In: Press A, editor. Washington D.C: 1st ed. ASM Press.
- Chuck SL, Sande MA (1989) Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med 321: 794-799.
- Waters L, Nelson M (2005) Cryptococcal disease and HIV infection. Expert OpinPharmacother 6: 2633-2644.
- Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, et al. (2001) Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis 33: 690-699.
- Moosa MYS, Coovadia YM (1997) Cryptococcal meningitis in Durban, South Africa: A comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV) - positive and HIV-negative patients. Clin Infect Dis24: 131-134.
- Mitchell TG, Perfect JR (1995) Cryptococcosis in the era of AIDS--100 years after the discovery of Cryptococcus neoformans. ClinMicrobiol Rev 8: 515-548.
- Bicanic T, Harrison TS (2005) Cryptococcal meningitis. Br Med Bull 72: 99-118.
- Feldmesser M, Harris C, Reichberg S, Khan S, Casadevall A (1996) Serum cryptococcal antigen in patients with AIDS. Clin Infect Dis 23: 827-830.
- Bindschadler DD, Bennett JE (1968) Serology of human cryptococcosis. Ann Intern Med 69: 45-52.
- CDC - Fungal Diseases - C. neoformanscryptococcosis. www.cdc.gov/fungal/cryptococcosis-neoformans. Accessed: 12/5/2014.
- Lortholary O, Poizat G, Zeller V, Neuville S, Boibieux A, et al. (2006) Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 20: 2183-2191.
- Venkateswarlu K, Taylor M, Manning NJ, Rinaldi MG, Kelly SL (1997) Fluconazole tolerance in clinical isolates of Cryptococcus neoformans. Antimicrob Agents Chemother 41: 748-751.
- Polak A (1992) 5-Fluorocytosine and its combination with other antifungal agents. In Yamaguchi H, Kobayashi GS, Takahashi H, eds. Recent Progress in Antifungal Chemotherapy. New York: Marcel Dekker 1992: 77–85.
- Joseph-Horne T, Hollomon D, Loeffler RS, Kelly SL (1995) Cross-resistance to polyene and azole drugs in Cryptococcus neoformans. Antimicrob Agents Chemother 39: 1526-1529.
- Ochieng PO, McLigeyo SO, Amayo EO, Kayima JK, Omonge EO (2009) Nephrotoxicity of Amphotericin B in the treatment of cryptococcal meningitis in axquired immunodeficiency syndrome patients. East Afr Med J. 86: 435–441.
- Trilles L, Fernández-Torres B, LazéraMdos S, Wanke B, Guarro J (2004) In vitro antifungal susceptibility of Cryptococcus gattii. J ClinMicrobiol 42: 4815-4817.
- Bovers M, Hagen F, Boekhout T (2008) Diversity of the Cryptococcus neoformans-Cryptococcus gattii species complex. Rev IberoamMicol 25: S4-12.
- Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmericanCryptococcal Study Group (2003) Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis 9: 189-195.
- Kwon-Chung KJ, Edman JC, Wickes BL (1992) Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun 60: 602- 605.
Citation: Duggal S, Duggal N, Hans C, Duggal AK (2014) Epidemiology of Cryptococcal Meningitis Associated with HIV in an Indian Hospital .Epidemiology (Sunnyvale) 4:166. Doi: 10.4172/2161-1165.1000166
Copyright: © 2014 Duggal S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited..
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 16878
- [From(publication date): 9-2014 - Apr 23, 2024]
- Breakdown by view type
- HTML page views: 12411
- PDF downloads: 4467