Evaluating Vigilance in Fibromyalgia through Objective Measures
Received: 02-Oct-2015 / Accepted Date: 07-Dec-2015 / Published Date: 10-Dec-2015
Abstract
Objective
Fibromyalgia syndrome (FS) involves chronic pain accompanied by alteration of cognitive functions, mainly memory and attention. Despite vigilance is a pre-requisite for appropriate performance in most cognitive tasks, it has not been assessed directly in fibromyalgia. The current research aimed to study vigilance in this illness and to explore the potential role of other common symptoms of the syndrome such as pain, sleep quality, anxiety and depression in vigilance performance.
Methods
The Psychomotor Vigilance Task (PVT) was used to assess the vigilance level in a group of fibromyalgia female patients (n=28) compared to a group of healthy women (n=18) matched in age and education level.
Results
The fibromyalgia group reported higher levels of anxiety, depression, negative mood and pain, poorer sleep quality, and lower levels of alertness than the control group. In the PVT, the fibromyalgia group showed slower responses in the PVT as compared to the control group. Fibromyalgia patients further showed high individual differences in vigilance, with a subgroup of them having similar performance in the PVT than the control group. Performance in the PVT showed no relationship with subjective measures.
Conclusion
These findings provided direct support to a vigilance deficit in fibromyalgia as a group. However, there are individual differences suggesting that not all the patients with fibromyalgia necessarily experience vigilance detriment. Since the vigilance state can determine basic functions such as memory and attention, these individual differences should be considered when assessing other cognitive domains in fibromyalgia.
Keywords: Alertness; Sustained attention; Vigilance; Anxiety; Sleep quality; Pain; PVT
Abbreviations
FS: Fibromyalgia Syndrome; PVT: Psychomotor Vigilance Task; RT: Reaction Time; SD: Standard Deviation; MPQ: McGill Pain Questionnaire; HAD: Hospital Anxiety and Depression Scale; PSQI: Pittsburgh Sleep Quality Index
Introduction
Fibromyalgia syndrome (FS) is a chronic pain disorder characterized by widespread musculoskeletal pain and diffuse tenderness, requiring responses of pain elicited by digital palpation of at least 11 out of 18 specified bilateral tender points for its diagnosis [1]. Although pain is the core feature of fibromyalgia, this syndrome is generally accompanied by many other complaints as fatigue, poor sleep quality, depressive and anxious symptoms, irritable bowel syndrome and cognitive dysfunction [2]. Regarding cognitive alteration, most complaints refer to memory and attentional problems where the main impairment appears in tasks involving controlled processing, such as working memory and executive function [3]. However, whereas there is broad research on memory and attention processes in fibromyalgia syndrome, studies focusing on vigilance are practically inexistent and knowledge on this issue is mainly based on inferences from tasks designed to study more complex cognitive functions. This topic of research is important for two reasons: (1) clinical research has shown sustained attention deficits associated with a variety of neurological disorders, such as attention deficit and hyperactivity disorder (ADHD) [4] or traumatic brain injury [5], (2) vigilance is a pre-requisite for appropriate performance in cognitive tasks, for example, demanding executive control [6]. Therefore, the current research aimed to study vigilance in fibromyalgia.
Attention deficits in fibromyalgia have been studied by the ANT-I task (Attentional Network Task for Interactions), developed by Callejas et al. [7] as an adaptation of the ANT [8]. The ANT assesses efficiency of the three attentional networks (alerting, orienting and executive control), while the ANT-I also measures the interactions between each other. When performing the ANT-I task, fibromyalgia patients show impairments in alerting and executive functioning, while the more automatic, orienting network, is preserved [9]. The deficit in executive control was confirmed by a subsequent study reporting deficits in temporal orienting and response inhibition in fibromyalgia [10]. This study also found that the overall reaction times were slower in the fibromyalgia group than in the control group, which was interpreted as a general deficit in vigilance.
There exists wide evidence supporting that coping with pain while performing a cognitive task consumes attentional resources, preventing the processing of other information and thus affecting performance [11]. However, in studies focusing on fibromyalgia the findings are heterogeneous. On one hand, Park et al. [12] and Suhr [13] found no relationship between pain, measured by the McGill Pain Questionnaire (MPQ) [14] and performance in attention and memory tasks. Landro et al. [15] assessed pain through a visual analogue scale (VAS) during the three days previous to the cognitive measurements and did not found a relationship between the two, either. On the other hand, pain has been related to task performance in studies that measured pain at the time of cognitive testing, by either a Numerical Rating Scale (NRS) [16] or an algometer [17].
Affective symptoms, mainly anxiety and depression, are also thought to influence cognitive performance in fibromyalgia syndrome, but the findings remain inconclusive. Whereas some studies did not find any relationship between affective and cognitive measures in fibromyalgia [12,18,19], others reported that anxiety, but not depression, was related to memory and concentration measures. By contrast, Suhr [13] and Septhon et al. [20] showed a relationship between depression symptoms and poor memory in fibromyalgia patients. Finally, Dick et al. [16] related both depression and anxiety symptoms to impaired working memory as measured by the Test of Everyday Attention (TEA) and the Reading Span Test (RST), but differences in cognitive performance between fibromyalgia patients and control participants yet remained when these affective symptoms were controlled for.
Additionally, FS involves important complaints referring sleep quality [1,21-22]. Vigilance has been studied in sleep disorders involving excessive daytime sleepiness like narcolepsy, insufficient sleep syndrome and hypersomnia [23] or obstructive sleep apnea [24,25]. In all these studies, daytime sleepiness was related to altered performance in the Psychomotor Vigilance Task (PVT), a simple reaction-time task specially designed for measuring vigilance. Sleep impairment has also been related to attentional detriment in fibromyalgia, evaluated through the ANT-I [26], but a specific evaluation of vigilance in FS was still missing in previous literature. In order to fill this gap, in the present study we employed the PVT in a group of fibromyalgia patients compared to a control group of healthy women. Beyond evaluating the general level of vigilance, this task allows detecting the vigilance decrement as reaction times (RTs) increase along time on task. In addition, we studied its possible relationship with self-reported physical (pain, sleep quality) and affective (depression, anxiety) symptoms. Similarly, as sleepiness [23-25] and depression [13,20] are likely to affect cognitive performance, we also tested the subjective level of alertness and mood state at the moment of accomplishing the task, and explored its relationship with performance in the PVT.
First we expected fibromyalgia patients to show a general deficit in vigilance compared with a matched control group. Then, we further explored which factors (sleep quality, anxiety, depression and subjective alertness and mood state) could mediate the impact over cognitive performance.
Methods
Participants
Forty-eight women (mean age: 50.72 years; SD: 6.67; range: 44-61) participated voluntarily in this study. The fibromyalgia group included thirty patients who met the following inclusion criteria: 1) being diagnosed with fibromyalgia syndrome according to the American College of Rheumatology criteria [1], 2) having significant selfreported insomnia according to the American Psychiatric Association (2000) [27], since sleep problems are related to worse vigilance performance [24-26], and 3) maintaining habitual medication regimen at least one month before and throughout the study. They were referred from the Rheumatology Service and Pain Unit of Virgen de las Nieves Hospital from Granada, Spain, to a cognitive-behavioural program carried out in the Clinic Psychology Unit from the Faculty of Psychology, intended to improve the symptoms of the patients (management of pain, cognitive and affective symptoms and those relating to sleep quality). The control group was composed of 18 healthy women matched in age and education level to the fibromyalgia patients. The education level could range from 0 (“no regulated education”) to 6 (University studies) and was considered to balance the groups. Exclusion criteria for both fibromyalgia and control groups were pregnancy, concomitant major medical conditions (e.g. inflammatory rheumatic diseases, uncontrolled endocrine disorders, cancer, etc.) including a medical history of important head injury, neurological disorder, or any serious major axis I diagnoses of the DSM-IV-TR (American Psychiatric Association, 2000) [27]. In addition, the control group was free from pain or sleep disorders and significant depressive or anxiety symptoms.
Data from one participant from the control group and two participants from the fibromyalgia group were excluded from the analysis as their mean reaction time (RT) was above 3 standard deviations from their group average in the PVT task.
Apparatus and stimulus
Psychological measures: All the participants completed a test battery in order to evaluate any relevant psychological, sleep-related and pain symptoms. The McGill Pain Questionnaire (MPQ, abbreviated version [14] in its Spanish version [28] assessed several dimensions of the pain experience by means of 15 verbal pain descriptors (sensorial and affective), a current pain index, and a visual analogue scale ranging from 1 (‘‘no pain’’) to 10 (‘‘extreme pain’’) regarding pain intensity in the last week. The Hospital Anxiety and Depression Scale (HAD) [29] in its Spanish version [30] was used to assess anxiety and depression symptoms through 14 items scoring from 0 to 3. The Pittsburgh Sleep Quality Index (PSQI) [31] in its Spanish version [32] explored seven dimensions of sleep quality: Subjective Sleep Quality, Sleep Latency, Sleep Duration, Habitual Sleep Psychology and Health Efficiency, Sleep Disturbances, Use of Sleeping Medication and Daytime Dysfunction, through 19 items. The total score ranged from 0 (“absence of disturbance”) to 21 (“severe disturbance”).
The levels of subjective alertness and mood of every participant just before completing the behavioural task were assessed by the Monk Visual Analogue Scale [33]. Participants had to place a mark on a line ranging from 0 to 100 to report their state on each of the following eight items: alert, sad, tense, effort, happy, weary, calm and sleepy. Scores on these items are summarised in two global dimensions, alertness and mood, with higher values meaning higher subjective alertness level and more positive mood state, respectively.
We were interested on these subjective measures as they were expected to be different in the control and fibromyalgia groups and they are likely to affect vigilance performance.
Behavioural tasks: The participants carried out the Psychomotor Vigilance Task (PVT) 1, programmed using the E-Prime software [34]. The PVT is a computerized visual reaction-time task that evaluates sustained attention. In the current version, the target stimulus was a red circumference with 8cm diameter (8.03° x 8.03° of visual angle at a viewing distance of 60 cm) over a black background. In every trial, the circumference started to fill itself with red colour after a delay interval ranging randomly from 2000 to 10000 ms, to which participants had to respond by pressing the space bar as quickly as possible. If the participant responded before 1500 ms, the RT was recorded and displayed to the participants as feedback. Otherwise, the trial was computed as a miss error and participants received feedback. Then, the next trial began. Finally, if participants responded before the circumference started to fill in, the trial was computed as anticipation error and the message “too early!” appeared. This task was presented for 10 min continuously.
Procedure
The fibromyalgia groups completed two sessions. The first session was a 1-2 h semi-structured interview about the onset and course of the symptoms, personal antecedents and psychological status. After the interview they were given a battery of questionnaires to be completed at home. In the second session the participants performed the cognitive tasks in the computer. The control group received only the experimental session and, after the experiment, they also completed at home the same set of questionnaires except for those regarding the evolution of the disease. Testing conditions were similar for all the participants.
Design and data analysis
Demographic and psychological measures: Age, education level and the scores of pain (MPQ), sleep quality (PSQI), trait anxiety (HAD Anxiety), depression (HAD-Depression) and subjective alertness and mood (Monk Visual Analogue Scale) were submitted to one-way ANOVAs with group as between-subjects factor with two levels (fibromyalgia, control).
Psychomotor Vigilance Task (PVT): The first trial, considered as a practice trial, and trials with RTs above 1000 ms (2.50% rejected) were not analysed. Mean RTs between 100 and 1000 ms were submitted to a mixed 2 x 2 ANOVA with group (fibromyalgia and control) manipulated between-subjects, and block (block 1, i.e. first five minutes of the task, block 2, last five minutes of the task) manipulated within-participants. Manipulating the block served to analyse the vigilance decrement in fibromyalgia. The proportions of anticipatory responses (RTs <100 ms) and lapses (RTs >500 ms) were similarly analysed.
Additionally, we performed linear correlation analyses between RTs in this task and age, education level and the scores in every questionnaire: pain (MPQ), sleep quality (PSQI), trait anxiety (HAD Anxiety), depression (HAD-Depression) and subjective alertness and mood (Monk Visual Analogue Scale).
Results
Demographic and psychological measures
No significant differences were found between the control group and fibromyalgia patients in age or education level (both Fs<1). In contrast, the fibromyalgia group significantly reported higher scores of pain, anxiety and depression, worse sleep quality, lower subjective alertness and more negative mood state than the control group (all ps<.01). See Table 1 for further details.
| Questionnaires | Control Group | Fibromyalgia Group |
|---|---|---|
| Age | 50.41 (6.26) | 51.50 (6.90) |
| Education level | 3.82 (1.7) | 3.48 (1.65) |
| MPQ | 2.25 (3.73) | 22.80 (7.72) |
| PSQI | 7.87 (5.24) | 13.93 (4.18) |
| HAD_Anxiety | 4.37 (4.03) | 11.11 (4.65) |
| HAD_Depression | 6.37 (3.20) | 10.29 (4.97) |
| Monk_Alertness | 70.91 (16.04) | 44.88 (17.11) |
| Monk_Mood | 71.92 (12.5) | 45.80 (17.08) |
Table 1: Mean and standard deviations of age, education level and scores on each questionnaire: McGill Pain Questionnaire (MPQ), Pittsburgh Sleep Quality Index (PSQI), Hospital Anxiety and Depression Scale (HAD) and Monk Visual Analogue Scale.
Behavioural results
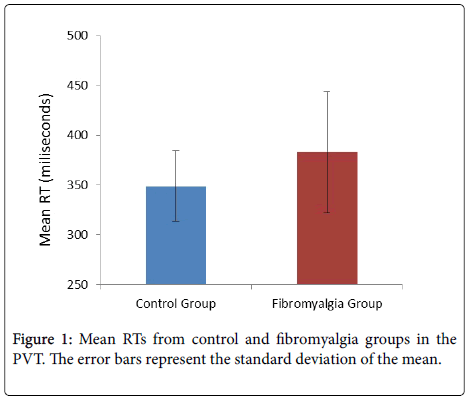
Psychomotor Vigilance Task (PVT): The 2 (group) × 2 (block) ANOVA on the mean RTs showed a significant effect of group, F(1, 43)=4.46, p=0.04, ηp2=0.09, with larger reaction times in the fibromyalgia group (mean=383 ms; SD=61 ms) compared with the control group (mean=349 ms; SD=36; see Figure 1). The main effect of block, F(1, 43)=15.32, p<0.01, ηp2=0.26, replicated the vigilance decrement, with longer RTs in the second block (378 ms; SD=98 ms) vs. the first block (362 ms SD=96). This RT decrement did not differ between groups (group x block: F...).
The ANOVA on the anticipation responses did not show any significant effects (all Fs<1). The ANOVA on the lapses showed a marginal effect of group: F(1, 43)=3.83, p=.06, that pointed to a tendency for more lapses in the fibromyalgia group (mean=9.89; SD=11.27) compared with the control group (mean=4.06; SD=3.19).
In order to investigate potential mediators of the vigilance deficit in fibromyalgia we computed linear correlations between the mean RTs in the PVT and both demographic and psychological measures including the MPQ, PSQI, HAD-Anxiety, HAD-Depression, subjective alertness and mood (Monk Visual Analogue Scale) in this group. However, the analyses did not show any significant effects (all ps > .18).
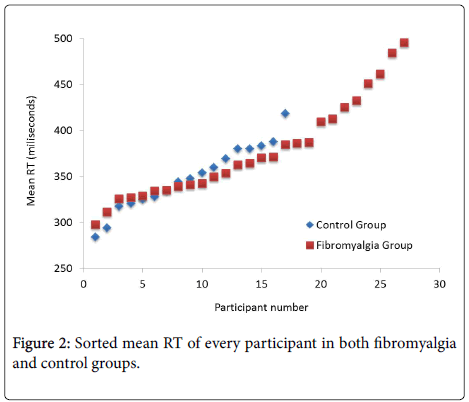
Finally, further inspection of descriptive statistics showed that the fibromyalgia group had larger variability than the control group in both mean RT (SD=61 ms vs. SD=36 ms; see also Figure 1) and lapses (SD=11.27 vs. SD=3.19). Figure 2 plots the sorted mean RTs for each group, which suggests similar performance in the vigilance task between participants in the control group and most of the patients (20 out of 28) in the fibromyalgia group.
This visual impression was analysed statistically by performing a median RT split procedure in both groups, leading to an ANOVA with group (low-vigilance fibromyalgia, high-vigilance fibromyalgia, low-vigilance control and high-vigilance control) as factor and mean RT as dependent variable.
The ANOVA yielded a main effect of group: F(3, 41)=26.09, p<.01, ηp2=0.66 (Figure 3). Specifically, the low-vigilance fibromyalgia group (mean=429 ms, SD=52) responded significantly slower than the remaining groups (all ps≤0.02). Surprisingly, the performances from both the high-vigilance fibromyalgia and high-vigilance control groups did not differ significantly, F(1, 21)=3.15, p=0.09. Also interestingly, the high-vigilance fibromyalgia group responded faster than the lowvigilance control group, F(1, 20)=25.68, p
Discussion
The study of cognitive alteration in fibromyalgia syndrome has generated increasing interest during the last years, mainly focusing in memory problems and executive dysfunction [3]. However, vigilance in fibromyalgia had been studied just indirectly through complex and long tasks designed for investigating other cognitive functions, such as the ANT-I task [9] or the temporal orienting and response inhibition task [10], where vigilance deficits were deduced from slower overall reaction times. Since our current goal was to measure vigilance directly, we employed a task specially designed for that purpose, the Psychomotor Vigilance Task (PVT). Despite its brief duration (just 10 minutes, avoiding to extenuate patients), this task is sensitive to different conditions expected to affect the vigilance level, as sleep deprivation [35-38], which is commonly associated with this illness. Thus, our aim was twofold: 1) to specifically study vigilance in fibromyalgia patients through an objective measure of vigilance as provided by the PVT, and 2) to explore the possible modulating factors of the vigilance state in these patients.
The results confirmed our hypothesis by showing slower reaction times on the PVT in fibromyalgia patients compared with the control group. This finding provides direct support to an alteration of vigilance in fibromyalgia, as suggested by Miro et al [9] and Correa et al [10] on the basis of larger overall reaction times in both ANT-I and temporal orienting tasks. However, the high variability in performance within the fibromyalgia group indicated that vigilance was not homogeneously altered among patients. Indeed, after dividing the groups according to their RTs, we found that the fibromyalgia group with higher vigilance level (i.e., faster reaction times in the PVT) showed similar performance to the high-vigilance control group, and even better performance than the control group with low vigilance. These results were unexpected according to the cognitive impairment attributed to the syndrome [3,9,10], that is, the high-vigilance fibromyalgia group should perform worse than any of the control groups. Instead, we found that not all fibromyalgia patients actually show vigilance alterations.
Moreover, in line with previous literature, our correlational analyses did not show clear associations between subjective measures and the results of the PVT in our fibromyalgia patients. Regarding depression and anxiety, the absence of correlation with performance group fits the results of several studies [12,18,19], while other research has provided mixed or inconclusive findings [13,16,20], leading to the general conclusion that the affective symptoms could explain only a modest proportion of the variability in cognitive performance.
Similarly surprising was our null finding of correlations between cognitive impairment and other symptoms of the illness such as pain or sleep disturbance. In healthy participants, frontal areas are involved in the maintenance of vigilance [39], and managing pain while performing cognitive tasks consumes some of the resources required for processing other information [11]. Since both pain processing and high-level cognitive functions show neural overlapping in frontal areas [11,40-45], we expected to find a correlation between pain scores and the reaction times in the PVT. However, this relationship has been mainly found in studies where pain was measured just at the time of cognitive testing [16,17]. Thus, a possible explanation for the absence of correlation in the present and other studies [12,13] could be that the MPQ measures pain retrospectively. Therefore, future studies should include on-line measures of pain, for example, by an algometer.
Likewise, poor sleep quality or sleep deprivation impairs cognitive performance in tasks involving the prefrontal cortex [37,46-48] and vigilance as measured by the PVT [35,36,45]. Although sleep disturbance is an important complaint of fibromyalgia patients, the role of sleep on cognitive performance in FS had not been fully considered. Cote and Moldofsky [48] found that an index of poor sleep quality (more proportion of time in stage 1 of sleep) could predict slow speed of performance in FS. However, several studies on the association between attention and memory performance and selfreported sleep quality [16,18] did not find a relationship, which is in line with our results. Future research should therefore use different measures of sleep quality (e.g. polysomnography) and test larger clinical samples.
Conclusion
Fibromyalgia is a highly complex syndrome including alterations in physical, cognitive and affective dimensions. Although pain receives most attention by patients and health professionals due to its aversive character, we cannot leave aside that all the other symptoms are potentially dysfunctional, especially if we consider that every alteration may impact on the rest. The vigilance function has been scarcely investigated, despite being a pre-requisite for appropriate performance in most cognitive tasks, so its deterioration might significantly affect everyday life activities. Here we show for the first time a vigilance deficit in fibromyalgia through direct objective measures. Nevertheless, we further found that vigilance was actually preserved in a proportion of fibromyalgia patients. This finding suggests that individual differences in vigilance state should be considered when testing or training cognitive functions in this population. According to previous studies, vigilance performance may be affected by other symptoms of the syndrome, but our correlational data were not conclusive on this regard. Further research is then necessary to clarify the role of each symptom in the whole, so that more selective interventions could improve the quality of life in fibromyalgia patients.
References
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160-172.
- Spaeth M, Briley M (2009) Fibromyalgia: a complex syndrome requiring a multidisciplinary approach. Hum Psychopharmacol 24 Suppl 1: S3-10.
- Glass JM (2009) Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum Dis Clin North Am 35: 299-311.
- Huang-Pollock CL, Nigg JT, Halperin, JM (2006) Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychology 20: 420-429.
- Sinclair KL, Ponsford JL, Rajaratnam SM, Anderson C (2013) Sustained attention following traumatic brain injury: use of the Psychomotor Vigilance Task. J ClinExpNeuropsychol 35: 210-224.
- Robertson IH, O’Connell R (2010) Vigilant attention. In Nobre AC &Coull JT (Eds.), Attention and Time (79-88).
- Callejas A, Lupiáñez J, Tudela P (2004) The three attentional networks: on their independence and interactions. Brain Cogn 54: 225-227.
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002) Testing the efficiency and independence of the attentional networks. J Cognitive Neurosci 14: 340–347.
- Miró E, Lupiáñez J, Hita E, Martínez MP, Sánchez AI, et al. (2011) Attentional deficits in fibromyalgia and its relationships with pain, emotional distress and sleep dysfunction complaints. Psychol Health 26: 765-780.
- Correa A, Miró E, Martínez MP, Sánchez AI, Lupiáñez J (2011) Temporal preparation and inhibitory deficit in fibromyalgia syndrome. Brain Cogn 75: 211-216.
- Eccleston C, Crombez G (1999) Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 125: 356-366.
- Suhr JA1 (2003) Neuropsychological impairment in fibromyalgia: relation to depression, fatigue, and pain. J Psychosom Res 55: 321-329.
- Melzack, R (1987) The short-form McGill Pain Questionnaire. Pain 30: 191-197.
- Landrø NI, Stiles TC, Sletvold H (1997) Memory functioning in patients with primary fibromyalgia and major depression and healthy controls. J Psychosom Res 42: 297-306.
- Dick BD, Verrier MJ, Harker KT, Rashiq S (2008) Disruption of cognitive function in fibromyalgia syndrome. Pain 139: 610-616.
- Munguía-Izquierdo D, Legaz-Arrese A, Moliner-Urdiales D, Reverter-Masía J (2008) (Neuropsychological performance in patients with fibromyalgia syndrome: relation to pain and anxiety). Psicothema 20: 427-431.
- Grace GM, Nielson WR, Hopkins M, Berg MA (1999) Concentration and memory deficits in patients with fibromyalgia syndrome. J Clin Exp Neuropsychol 21: 477-487.
- Verdejo-García A, López-Torrecillas F, Calandre EP, Delgado-Rodríguez A, Bechara A (2009) Executive function and decision-making in women with fibromyalgia. Arch Clin Neuropsychol 24: 113-122.
- Sephton SE, Studts JL, Hoover K, Weissbecker I, Lynch G, et al. (2003) Biological and psychological factors associated with memory function in fibromyalgia syndrome. Health Psychol 22: 592-597.
- Mease P, Arnold LM, Choy EH, Clauw DJ, Crofford LJ, et al. (2009) Fibromyalgia syndrome module at OMERACT 9: domain construct. J Rheumatol 36: 2318-2329.
- Prados G, Miro E (2012) [Fibromyalgia and sleep: a review]. Rev Neurol 54: 227-240.
- Thomann J, Baumann CR, Landolt HP, Werth E (2014) Psychomotor vigilance task demonstrates impaired vigilance in disorders with excessive daytime sleepiness. J Clin Sleep Med 10: 1019-1024.
- Sforza E, Haba-Rubio J, De Bilbao F, Rochat T, Ibanez V (2004) Performance vigilance task and sleepiness in patients with sleep-disordered breathing. Eur Respir J 24: 279-285.
- Kim H, Dinges DF, Young T (2007) Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep 30: 1309-1316.
- Miró E, Lupiáñez J, Hita E, Martínez MP, Sánchez AI, et al. (2011) Cognitive-behavioral therapy for insomnia improves attentional function in Fibromyalgia Syndrome: a pilot randomized controlled trial. J Health Psychol 16: 770-780.
- American Psychiatric Association. (2000) Diagnostic and statistical manual of mental disorders. (4th ed. Text Revision). American Psychiatric Association; Washington, DC.
- Lázaro C, Caseras X, Whizar-Lugo VM, Wenk R, Baldioceda F, et al. (2001) Psychometric properties of a Spanish version of the McGill Pain Questionnaire in several Spanish-speaking countries. Clin J Pain 17: 365-374.
- Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361-370.
- Herrero MJ, Blanch J, Peri JM, De Pablo J, Pintor L, et al. (2003) A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry 25: 277-283.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28: 193-213.
- Royuela A,Macías JA (1997). Propiedadesclinimétricas de la versióncastellana del cuestionariode Pittsburgh. Vigilia-Sueño 9: 81-94.
- Monk TH (1989) A Visual Analogue Scale technique to measure global vigor and affect. Psychiatry Res 27: 89-99.
- Schneider W, Eschmann A, Zuccolotto A (2002) E-prime reference guide. Pittsburgh, PA. Psychology Software Tools.
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, et al. (1997) Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep 20: 267-277.
- Tucker AM, BasnerRC, Stern Y, Rakitin BC (2009) The variable response-stimulus interval effect and sleep deprivation: an unexplored aspect of psychomotor vigilance task performance. Sleep 32: 1393-1395.
- Horne JA (1993) Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry 162: 413-419.
- Drummond SP, Brown GG (2001) The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology 25: S68-73.
- Drummond SP1, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, et al. (2005) The neural basis of the psychomotor vigilance task. Sleep 28: 1059-1068.
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, et al. (2004) Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127: 835-843.
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, et al. (2007) Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci 27: 4004-4007.
- Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T (2008) Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain 131: 3222–3231.
- Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, et al. (2009) Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med 71: 566-573.
- Solberg Nes L, Roach AR, Segerstrom SC (2009) Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med 37: 173-183.
- Jewett ME, Dijk DJ, Kronauer RE, Dinges DF (1999) Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep 22: 171-179.
- Drummond SP, Gillin JC, Brown GG (2001) Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res 10: 85-92.
- Durmer JS, Dinges DF (2005) Neurocognitive consequences of sleep deprivation. SeminNeurol 25: 117-129.
- Altena E, Van Der Werf YD, Sanz-Arigita EJ, Voorn TA, Rombouts SA, et al. (2008) Prefrontal hypoactivation and recovery in insomnia. Sleep 31: 1271-1276.
- C-oté KA, Moldofsky H (1997) Sleep, daytime symptoms, and cognitive performance in patients with fibromyalgia. J Rheumatol 24: 2014-2023.
Citation: Morilla BR, Lupianez J, Miro E, Martinez MP, Sanchez AI, et al. (2015) Evaluating Vigilance in Fibromyalgia through Objective Measures. Fibrom open 1:102.
Copyright: ©2015 Morilla BR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 9895
- [From(publication date): 12-2016 - Apr 25, 2024]
- Breakdown by view type
- HTML page views: 9252
- PDF downloads: 643