Iodine-123 Ioflupane Scintigraphy for the Diagnosis of Lewy Body Disease Presenting as a Posterior Cortical Atrophy
Received: 25-Apr-2016 / Accepted Date: 12-May-2016 / Published Date: 18-May-2016 DOI: 10.4172/2472-095X.1000110
Abstract
We report the case of a 80-year old man who was admitted for loss of autonomy due to repeated falls and cognitive decline. The only symptom reported by the patient was difficulties with judging distances. Neurological examination revealed a transient extrapyramidal syndrome due to treatment with risperidone for visual hallucinations, but more importantly, a marked impairment of higher visual functions. Computerized tomography showed bilateral occipital lobe atrophy. Since Lewy body disease is a rare cause of Posterior Cortical Atrophy and given the visual hallucinations, we performed a 123-I-ioflupane brain scintigraphy which confirmed the diagnosis. To our knowledge, this is the first application of ioflupane-single photon emission computerized tomography in the diagnostic workup of a patient with Benson’s syndrome.
Keywords: Dementia; Posterior cortical atrophy; Lewy body disease; Iodine-123-ioflupane; Striatal imaging
Introduction
Posterior Cortical Atrophy (PCA) or Benson’s syndrome is a rare progressive clinico-radiological entity, characterized by the clinical consequences of an atrophy of the primary visual cortex (occipital), as well as the dorsal (occipitoparietal, “where?”) and ventral (occipitotemporal, “what?”) visual streams (for a recent review, see Beh and coworkers) [1]. Problems start typically in the late sixth, early seventh decade, and patients present initially with–often vague-visual complaints due to disturbed high-order visual processes, in the absence of significant ophthalmological abnormalities. Most prominent features of PCA are elements of Balint’s syndrome (simultanagnosia, ocular apraxia and optic ataxia) and of Gerstmann’s syndrome (finger agnosia, right-left confusion, agraphia and acalculia), but also spatial disorientation, visual agnosia, alexia, anomia, apraxia, prosopagnosia, hemineglect or transcortical sensory aphasia.
PCA is often considered as an atypical form of Alzheimer’s disease (AD), since most cases present findings compatible with this diagnosis, either because of post-mortem evidence of plaques and tangles or of in vivo evidence of abnormal amyloid burden, as measured by amyloid positron emission tomography (PET) imaging or cerebrospinal fluid (CSF) biomarker analysis [2-4]. However, in some cases, pathological studies have found alternative diagnoses, such a prion diseases, corticobasal degeneration (CBD), subcortical gliosis or Lewy body disease (LBD) [2,3].
In this paper, we present the case of an 80-year old man suffering from dementia with prominent abnormalities of higher visual function. He fulfilled not only the proposed standardized diagnostic criteria of PCA, but also the diagnostic criteria of DLB. Iodine 123-ioflupane single photon emission computerized tomography (SPECT) imaging was used to confirm our diagnostic hypothesis.
Case Presentation
Clinical presentation
An 80-year old former graphic designer was admitted at the geriatric department for assessment of repeated falls. He had a history of a pacemaker for atrial fibrillation, right bundle branch block, hypertension, multinodular thyroid goiter, bilateral total hip arthroplasty, and bilateral glaucoma. Three months before admission, he was diagnosed with prostatic adenocarcinoma (T2N0M0) and treated with radiotherapy and hormones.
His wife reported the presence of complex visual and auditory hallucinations since several years, the patient seeing criminals and dangerous animals in his bedroom, especially at night, but without loss of autonomy for activities of daily life. One year before admission, he had stopped cooking his own meals. After radiotherapy, he had developed walking difficulties with lack of balance and coordination that led to falls, insomnia, mood swings, pathological laughter and crying. Cognitive decline had only recently led to a loss of autonomy for driving, writing, drawing and the management of financial and administrative tasks. One month before admission he had been hospitalized in another institution. No specific aetiology for his cognitive deterioration had been found, but a treatment with risperidone 1 mg had been initiated. This had been followed by further walking difficulties and loss of autonomy.
When asked why he was hospitalized, the patient spontaneously reported “difficulties with judging distances”.
General physical examination revealed atrial fibrillation (67 beats per minute) with normal blood pressure, implanted pacemaker, quadriceps amyotrophy, a lot of superficial wounds and hematomas, and orthostatic hypotension. Body mass index was 20. Neurologic examination at admission revealed an extrapyramidal syndrome characterized by a masked face, bradykinesia with a monotonous speech, permanent slow rest tremor, plastic rigidity with cogwheel phenomenon and a postural instability. After withdrawal of risperidone, this extrapyramidal syndrome and the walking difficulties disappeared rapidly and completely. He had no pyramidal or cerebellar signs, no sensory deficit and intact cranial nerves. Bedside cognitive testing was marked by profound visual high order impairment, and showed a fluctuating left-sided extracorporeal hemineglect, profound left optic ataxia, visual and tactile (but no auditory) agnosia, simultanagnosia (as tested with Navon’s figures but not on the Boston Cookie Theft Picture), color simultanagnosia and finger agnosia. On the other hand, he was capable of recognizing the faces of staff members. The patient showed a curious form of dysgraphia; writing sentences with an imaginary ballpoint which he put down afterwards or handed back to the examiner (see video clips in the Supplementary Material). Speech and reading were normal, and the patient was capable of spelling words backwards. He was disoriented in time and space, and was unable to find his room after only a few steps outside the door. He had dyscalculia, ideomotor apraxia and an impaired longterm memory. Ophthalmological examination revealed a long distance visual acuity of 20/32 for the right eye and 20/50 for the left and normal intra-ocular pressure with usual treatment.
Laboratory findings
Routine blood analysis was normal except for signs of malnutrition (prealbumin: 0.12 g/l ) and vitamin D deficiency. Viral and bacterial samplings were negative, as well as serum Para neoplastic and auto-immune antibodies. CSF analysis showed normal proteins, glucose and cell count normal biomarkers for AD (amyloid β-42, total tau and phosphorylated tau protein) and was negative for malignant cells and protein 14-3-3.
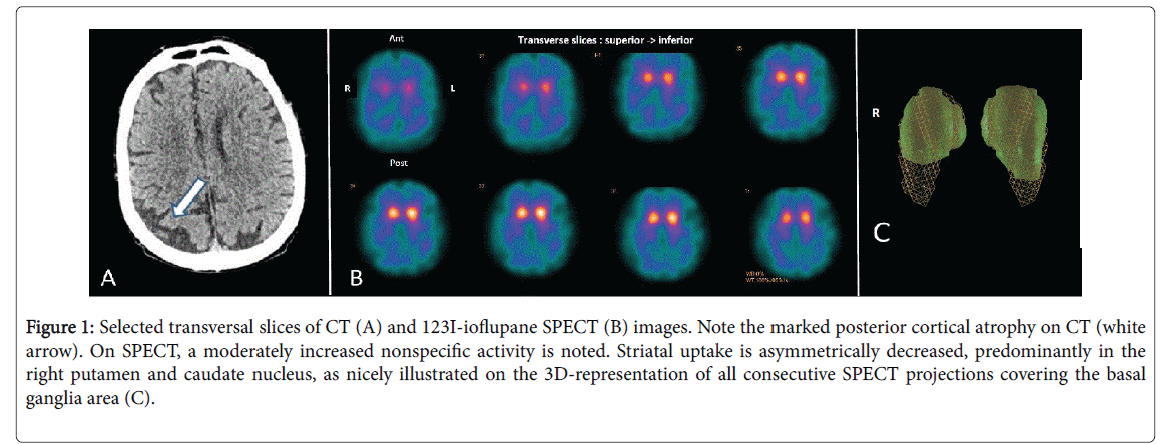
Magnetic resonance imaging was contra-indicated due to the pacemaker, but a computerized tomodensitometry (CT) of the brain showed marked bilateral atrophy of the occipital cortex, predominantly on the right side (Figure 1A). 123I-ioflupane SPECT revealed a bilaterally decreased uptake of both putamen and caudate nuclei, also predominant on the right side (Figures 1B and 1C).
Figure 1: Selected transversal slices of CT (A) and 123I-ioflupane SPECT (B) images. Note the marked posterior cortical atrophy on CT (white arrow). On SPECT, a moderately increased nonspecific activity is noted. Striatal uptake is asymmetrically decreased, predominantly in the right putamen and caudate nucleus, as nicely illustrated on the 3D-representation of all consecutive SPECT projections covering the basal ganglia area (C).
Evolution
The patient presented a substantial improvement in cognition and nutrition after 3 weeks of geriatric multidisciplinary stimulation. Hallucination’s frequency, sleep quality, anxiety and mood were significantly improved after 2 weeks of trazodone treatment. Vitamin D deficiency was corrected. An improvement in walking was noted and no falls were observed after admission. A return to home was organized with optimal home support and treatment with donepezil (5 mg once daily) was started (Figure 1).
Discussion
Our patient presented all cardinal features of the standardized diagnostic criteria of PCA, as proposed by Beh and co-workers: onset was insidious and progressive, visual impairment was prominent, with many signs of complex visual dysfunction, in the absence of stroke or tumor and with a relatively intact ophthalmological examination, insight and memory [1]. Moreover, he showed ideomotor apraxia, which is one of the five suggested supportive features.
The literature suggests that almost all cases of PCA are a visual forms of AD [5,6]. In a series of 100 consecutive patients with various focal lobe degenerations, all seven patients with PCA had AD as the primary neuropathology at autopsy, and none of them had Lewy bodies. The PCA group was the only variant of focal lobe degenerations were all patients had AD pathology [6]. Our patient had normal CSF concentrations of tau, phospho-tau and β42-amyloid. Although this pleads against AD as the etiological substrate, it cannot exclude it entirely. Indeed, in a series of 11 patients with PCA, all had abnormal amyloid PET scans, although 2 patients had 3 normal CSF biomarkers, which suggests that amyloid-PET is more sensitive than CSF sampling [4].
Other possible etiologies of PCA are CBD and Creutzfeldt-Jakob disease, but after risperidone withdrawal, our patient had no suggestive clinical features of CBD and CSF 14-3-3 protein was negative. He had only one core feature of the revised criteria for LBD (recurrent complex visual hallucinations), that led to the suspicion of LBD. 123I-ioflupane SPECT proved to be definitely pathological, which is a suggestive feature of LBD. The presence of one core and one suggestive feature permits the diagnosis of probable LBD [7].
Other authors have evoked the possibility of LBD as the primary etiological substrate for PCA, and Lewy bodies were found together with AD pathology in two brain autopsies [8-10]. In a retrospective analysis of 59 patients with PCA, 13 patients had visual hallucinations. These patients tended to be older and had more often rapid eye movement behaviour disorder (RBD), spontaneous (i.e., not drug induced) Parkinsonism or myoclonic jerks than patients without hallucinations. All 13 patients met the diagnostic criteria for LBD [8].
LBD might thus be the second most common etiology of PCA. To our knowledge, our case is the first in which 123I-ioflupane SPECT was performed and contributed to the diagnosis of LBD.
Conclusion
123I-ioflupane SPECT could be a valuable non-invasive tool for non-invasive in vivo diagnosis of LBD in patients with PCA. This is of clinical relevance, given the widely known potential harmfulness of neuroleptics in LBD and the potential benefit of treatment with acetylcholinesterase inhibitors [11-13]. LBD should be suspected in patients with PCA who are older and present with complex visual hallucinations, spontaneous Parkinsonism, myoclonic jerks or RBD.
References
- Beh SC, Muthusamy B, Calabresi P, Hart J, Zee D, et al. (2015) Hiding in plain sight: a closer look at posterior cortical atrophy. Pract Neurol 15:5-13.
- Crutch SJ, Schott JM, Rabinovici GD, Boeve BF, Cappa SF, et al. (2013) Shining a light on posterior cortical atrophy. Alzheimers Dement 9: 463-465.
- Seguin J, Formaglio M, Perret-Liaudet A, Quadrio I, Tholance O, et al. (2011) CSF biomarkers in posterior cortical atrophy. Neurology 76: 1782-1788.
- Beaufils E, Ribeiro MJ, Vierron E, Vercouillie J, Dufour-Rainfray D, et al. (2014) The pattern of brain amyloid load in posterior cortical atrophy using (18) F-AV45: Is amyloid the principal actor in the disease? Dement Geriatr Cogn Dis Extra 4:431-441.
- Goldstein MA, Ivanov I, Silverman ME (2011) Posterior cortical atrophy: an exemplar for renovating diagnostic formulation in neuropsychiatry. Compr Psychiatry 52:326-333.
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J,et al. (2007) Focal cortical presentations of Alzheimer's disease. Brain 130: 2636-2645.
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, et al. (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863-1872.
- Josephs KA, Whitwell JL, Boeve BF, Knopman DS, Tang-Wai DF, et al. (2006) Visual hallucinations in posterior cortical atrophy. Arch Neurol 63:1427-1432.
- Renner JA, Burns JM, Hou CE, McKeel DW Jr, Storandt M, et al. (2004) Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology 63: 1175-1180.
- Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, et al. (2003) Coexistent Lewy body disease in a case of visual variant of Alzheimer's disease. J Neurol Neurosurg Psychiatry 74:389.
- Bhasin M, Rowan E, Edwards K, McKeith I (2007) Cholinesterase inhibitors in dementia with Lewy bodies: a comparative analysis. Int J Geriatr Psychiatry 22:890-895.
- O’Brien JT, Mckeith IG, Walker Z, Tasch K, Booji J, et al. (2009) Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry 194:34-39.
- Goto H, Ishii K, Uemura T, Miyamoto N, Yoshikawa T, et al. (2010) Differential Diagnosis of Dementia with Lewy Bodies and Alzheimer Disease Using Combined MR Imaging and Brain Perfusion Single-Photon Emission Tomography. AJNR Am J Neuroradiol 31: 720-725.
Citation: Segers K, Benoit F, Duez M, Meyts JM, Hambye AS, et al. (2016) Iodine-123 Ioflupane Scintigraphy for the Diagnosis of Lewy Body Disease Presenting as a Posterior Cortical Atrophy. J Neuropsychopharmacol Mental Health 1: 110. Doi: 10.4172/2472-095X.1000110
Copyright: © 2016 Segers K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 11738
- [From(publication date): 6-2016 - Apr 25, 2024]
- Breakdown by view type
- HTML page views: 11016
- PDF downloads: 722