Lack of AMACR Immunostaining is an Independent Predictor of Poor Prognosis in Colorectal Carcinoma
Received: 24-May-2016 / Accepted Date: 16-Jun-2016 / Published Date: 17-Jun-2016 DOI: 10.4172/2161-0681.1000279
Abstract
Background: AMACR (Alfa-Methylacyl-CoA Racemase) overexpression has become a useful biomarker of prostate cancer. In the present cohort we are aiming to analyse AMACR immunostaining in normal colonic mucosa, colorectal adenoma, and colorectal carcinoma (CRC) to explore the significance of immune-staining in relation to clinic-pathological features, prognosis, and survival.
Materials and Methods: The study included 38 normal colonic mucosae, 40 colorectal adenomas, 196 CRC, and 49 associated lymph node metastasis. Tissue microarrays were designed and constructed and immunostaining was done using anti-AMACR antibody. Results: AMACR was absent in normal colonic mucosa while it showed positive immunostaining in 47.5% of adenomas, 53.6% colorectal carcinomas and 36.7% of nodal metastasis. There was no statistically significant difference between AMACR immunostaining in primary CRC in relation adenomas, and nodal metastasis. Low AMACR immunostaining showed significant association with the occurrence nodal metastasis (p=0.039) and distant metastasis (p=0.022). There was no significant association between AMACR immune-staining and other clinic pathological parameters. Regression analysis revealed that reduced AMACR immunostaining was an independent predictor of positive surgical resection margins, presence of Lymph vascular invasion, distant metastasis, and lymph node metastasis. AMACR immunostaining was not related to both diseases free survival and overall survival. Conclusion: AMACR immune-staining correlated with nodal metastasis and distant metastasis. Loss of immunostaining of AMACR is an independent predictor of lymph vascular invasion, positive surgical margin, nodal and distant metastasis. AMACR may serve as biomarker of progression and prognosis of CRC.
Keywords: AMACR; Immunohistochemistry; Colorectal carcinoma; Prognosis
Abbreviations
AJCC: American Joint Committee on Cancer; AMACR: Alfa- Methylacyl-CoA Racemse; CRC: Colorectal carcinoma
Introduction
Colorectal carcinoma (CRC) is a major cause for morbidity and mortality globally [1]. CRC is related to genetic and environmental factors. Epidemiological studies suggested obesity, diabetes mellitus, smoking, alcohol intake, and dietary habits as environmental factors contributing to CRC [2]. Low fibre diet and increased consumption of fatty acids are proposed the most contributing factors. The main source of branching fatty acids in humans is red meat and dairy products [3-5] Prolonged meat and fatty meals consumption increases the risk of incidence of CRC to 20-30% and is also linked to an increased incidence of mortality in CRC patients [3]. Some reports suggested that fatty acid may promote human carcinogenesis through stimulation of colonic cell proliferation or through genetic mutations that may affects enzymes responsible for detoxification of harmful metabolites resulting from its consumption [4,5].
AMACR (Alfa-Methylacyl-CoA Racemse) was identified in rat liver as mitochondrial and peroxisomal enzyme. It catalyses a key step in the metabolism of fatty acids and fatty acid derivatives via conversion of (2R) fatty acids into their S-stereoisomers, which can be further metabolised and degraded in order to produce energy. An increase in utilisation of energy from fat is the hallmark of many cancer cells [6,7]. AMACR immunostaining was detected in normal hepatocytes, epithelial cells of renal tubules, bronchial epithelium, salivary glands and gall bladder, whereas, it was absent in normal epithelium of prostate, lung, colon, skin, ovary and endometrium [8,9]. Severe reduction of AMACR activity is associated with neurological disorder due to accumulation of R-2-methyl fatty acids [10]. On the other hand, the consistent overexpression of AMACR in high grade intraepithelial neoplasia of prostate and primary and metastasising prostatic carcinoma pointed to its role in the biology of prostatic cancer [11-14]. Further studies revealed AMACR is overexpressed in other organ cancers as colon [15,16], liver [17], lung [18], bladder [19], lymphoma [20], renal cell carcinoma [21], and melanoma [22]. AMACR is rarely expressed in gastric [23], ovarian [24], and breast [25] carcinomas.
Despite the above promising data, there is still limited information about the relation of AMACR immune-staining to clinic-pathological variables and prognostic parameters of CRC. Exploration of characteristic AMACR immunostaining may add to understanding of mechanisms by which diet affects cancer colon development and may afford new insights into cancer prognosis, and therapy. The aim of the present study is to analyse AMACR immunostaining in normal, dysplastic and malignant colorectal tissues, and to explore significance of immunostaining in relation to clinic-pathological variables and free survival period.
Materials and Methods
Patients
The material of the present study represent paraffin blocks from 38 normal colonic mucosa, 40 colorectal adenoma, 196 colorectal carcinoma cases and 49 lymph nodes with metastatic CRC. Paraffin blocks were retrieved from database of the Department of Pathology, King Abdulaziz University from 1995 to 2010. Patients’ clinical information, follow up data and treatment outcome data were collected from hospital patients records. The following Clinicopathological parameters for CR adenomas and CRC cases were used; age, sex, tumour site and size, histological type and grade, stage at time of diagnosis (following the AJCC staging system) [26], margins of excision, and the presence of distant metastasis. Details of clinicpathological findings are listed (Table 1).
| Parameter | Number (%) | |
|---|---|---|
| Age | <60 years | 100 (51%) |
| ≥60 years | 96 (49%) | |
| Sex | Male | 109 (55.6%) |
| Female | 87 (44.4%) | |
| Grade | Well-differentiated | 40 (20.4%) |
| Moderately-differentiated | 129 (65.8%) | |
| Poorly-differentiated | 27 (13.8%) | |
| Tumour location | Right colon | 57 (29.1%) |
| Left colon | 116 (59.2%) | |
| Rectum | 23 (11.7%) | |
| Tumour size | <5cm | 88 (44.9%) |
| ≥ 5cm | 108 (55.1%) | |
| Primary tumour | T1 | 4 (2%) |
| T2 | 28 (14.3%) | |
| T3 | 145 (74%) | |
| T4 | 19 (9.7%) | |
| Lymphovascular invasion | Negative | 169 (86.2%) |
| Positive | 27 (13.8%) | |
| Margin status | Free | 185 (94.4%) |
| Involved | 11 (5.6%) | |
| Nodal metastasis | Negative | 104 (53.1%) |
| Positive | 85 (43.3%) | |
| Not applicable | 7 (3.6%) | |
| Distant metastasis | Negative | 139 (70.9%) |
| Positive | 57 (29.1%) | |
| Survival | Alive | 140 (71.4%) |
| Died of disease | 42 (21.4%) | |
| Data not available | 14 (7.2%) | |
| Relapse | Negative | 119 (60.7%) |
| Positive | 77 (39.3%) | |
Table 1: Clinicopathological parameters of CRC cases.
T1: Tumour invades sub-mucosa; T2: Tumour invades muscularis propria; T3: Tumour invades through the muscularis propria into the sub-serosa or into non-peritonealised pericolic or perirectal tissues; T4: Tumour directly invades other organs or structures, and/or perforates visceral peritoneum
The study was approved by the Research Committee of the Biomedical Ethics Unit, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia. All patients included in this study gave an informed written consent for utilisation of their material in research and was accepted by Research Committee of the Biomedical Ethics Unit.
Tissue microarray and immunohistochemistry
Paraffin blocks were sliced and stained routinely with haematoxylin and eosin to review histopathological characteristics of cases as histological type and grade, presence of lymph-vascular and perineural invasion, status of surgical margins, as well lymph node status. Using an automated microarrayer (Master 3D Histech) [27], two replicate tissue cores (1.5 mm each) were sampled and inserted into tissue microarray blocks. Blocks were sliced into 4 micron meter sections, embedded in sialinated slides in order to be stained by immunohistochemical staining. Immunostaining of AMACR was performed using Avidin-Biotin procedure following manufacturer’s kit instructions. Sections were stained immunohistochemically with primary antibody (mouse polyclonal antihuman AMACR antibody, 1:200 dilutions, Dako cytomation, Norden, Glostrup, Denmark) using automated immune-stainer (Ventana, Benchmark-XT). For each test, positive controls were selected from malignant prostatic tissue previously known to stain for AMACR, and as negative control we replaced primary antibody by Tris buffered saline. Immunohistochemical stain was evaluated semi-quantitatively by two separate expert pathologists (EE and WG) by counting the extent (%) of staining of AMACR in cells. The extent Immunostaining was scored as follows; negative when positivity is found in less than 10% of cells, low immunostaining (10-15% positivity), and high immunostaining when positivity is detected in more than 50% of cells.
Statistical analysis
Statistical analysis was performed using SPSS® Release 16.0. Statistical significance was determined at p value ≤ 0.05 and was 2- sided. The following tests were used. Mann Whitney test and Kruskal Wallis test to compare two and three groups of variables respectively. One sample non-parametric chi-square tested variance along one variable. Binary logistic regression analysis was used to prediction of lymph noel metastasis, distant metastasis, surgical resection margins involvement, lymph-vascular invasion, and local disease recurrence in relation AMACR immunostaining. The Kaplan-Meier procedure was used for disease-free and overall survival probabilities.
Results
AMACR immunostaining profile
AMACR immunostaining was shown exclusively in the cytoplasm of atypical and malignant cells. All normal colonic mucosa were negative to AMACR staining. 47.9% of colonic adenomas showed low positivity to AMACR for CRC cases, 42.9% showed low immunostaining profile while 10.7% of cases showed strong immunostaining (Table 2).
| Tissue | (n) | AMACR immunostaining | p value* | ||
|---|---|---|---|---|---|
| Negative immuno staining | Low immuno staining | High immuno staining | |||
| Normal colonic mucosa | 38 | 38 (0%) | 0 (0%) | 0 (0%) | <0.001 |
| Colorectal adenoma | 40 | 21 (52.5%) | 19 (47.5%) | 0 (0%) | 0.752 |
| Colorectal carcinoma | 196 | 91 (46.4%) | 84 (42.9%) | 21 (10.7%) | <0.001 |
| Lymph node metastasis | 49 | 31 (63.3%) | 16 (32.7%) | 2 (4.1%) | <0.001 |
Table 2: Distribution of scoring categories of AMACR immunostaining in different tissues examined.
n = number of cases falling in each category; *One sample nonparametric chi-square test
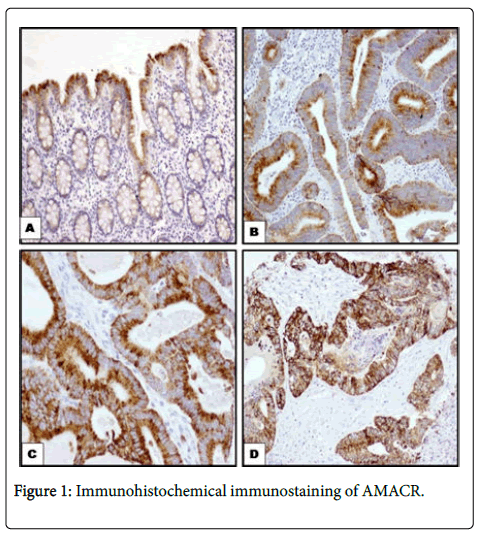
AMACR was downregulated in primary tumours (p< 0.001) and in nodal metastasis (p< 0.001). However, in adenoma there was no difference between low and high immunostaining (Figure 1).
A: Normal colonic mucosa showing focal staining in cytoplasm of surface epithelium only (100 x); B: Colorectal adenoma showing weak cytoplasmic expression (100x); C: Primary CRC carcinoma showing diffuse strong cytoplasmic immunoreactivity (100x); D: CRC metastasising to lymph nodes showing strong cytoplasmic staining (100x). Immunohistochemical labelling of AMACR was using anti- AMACR antibody. Diaminobenzidine was used as a chromogen and haematoxylin as a counterstain.
There was no statistically significant difference between AMACR immunostaining in primary CRC in relation to normal colonic mucosa, adenoma, and nodal metastasis (Table 3).
| p value* | |
| Malignant vs. Normal mucosa | 1.000 |
| Malignant vs.Adenoma | 0.283 |
| Malignant vs.Nodal metastasis | 0.646 |
| Nodal metastasis vs.Normal mucosa | 1.000 |
| Nodal metastasis vs.Adenoma | 0.974 |
| Adenoma vs.Normal mucosa | 1.000 |
Table 3: Differences in AMACR immunostaining in different tissues examined; *Mann-Whitney Test.
The relationship between AMACR Immuno-staining and clinic-pathological features of CRCs
Low AMACR immune-staining in CRC is associated with the occurrence of lymph node metastasis and distant metastasis. However, there was no significant association between AMACR immunostaining and age, sex, degree of differentiation, depth of tumour invasion, stage,lymph-vascular invasion, and status of surgical resection margins (Table 4).
| p value | |
|---|---|
| Age | 0.430✪ |
| Sex | 0.882✪ |
| Grade | 0.835* |
| Tumour location | 0.482 * |
| Tumour size | 0.834✪ |
| Depth of invasion (pT) | 0.452* |
| Lymphovascular invasion | 0.689✪ |
| Margin status | 0.238✪ |
| Nodal metastasis | 0. 039✪ |
| Distant Metastasis | 0.022✪ |
| Relapse | 0.195✪ |
Table 4: AMACR immunostaining in relation to clinicopathological parameters, * Kruskal-Wallis Test, ✪Mann-Whitney test.
Regression analysis revealed that AMACR downregulation was an independent predictor of surgical resection margins, lymphovascular invasion, nodal metastasis, and distant metastasis (Table 5).
| Variable | ExpB | 95% CI for ExpB | p value |
|---|---|---|---|
| Surgical resection margins | 0.067 | 0.036-0.123 | <0.001 |
| Lymph-vascular invasion | 0.182 | 0.121–0.275 | <0.001 |
| Nodal Metastasis | 2.9109 | 1.019–8.303 | 0.046 |
| Distant metastasis | 0.354 | 0.141–0.885 | 0.026 |
| Relapse | 0.550 | 0.222–1.367 | 0.198 |
Table 5: Binary logistic regression analysis for AMACR immunostaining.
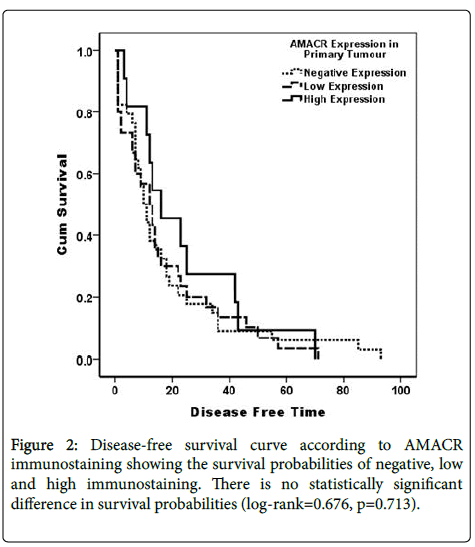
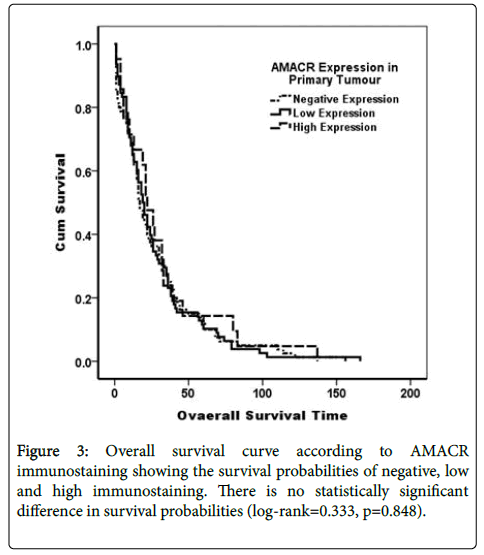
AMACR immunostaining was not related to both diseases free survival Log Rank (Mantel-Cox)=0.676, p=0.414, and overall survival Log Rank (Mantel-Cox)=0.330, p=0.566 (Figures 2 and 3).
Discussion
The biological role of AMACR overexpression is proved in carcinogenesis of many organ systems most probably due to its relation to beta-oxidation of branching fatty acids [7]. The greatest expression of AMACR was reported in prostatic and colonic carcinoma accounting for 92% and 83% respectively [11,15]. Nowadays, AMACR overexpression is considered a promising biomarker to distinguish normal prostate from malignant tissue particularly in ambiguous needle prostatic biopsies and even in blood samples [11,28]. The differential expression of AMACR in some malignancies rather than others, make it useful diagnostic tool in contrasting carcinoma originating from various primaries as colon, ovary and breast [29], and endometrial carcinomas [30]. Recently, attention was paid to AMACR as biomarker in preneoplastic conditions in many organs. Many studies reported significant correlation between AMACR expression and degree of dysplasia in preneoplastic conditions of gastrointestinal tract as Barrett’s esophagus and inflammatory bowel disease and suggested AMACR as additional risk factor in colorectal preneoplastic conditions and considered it as candidate adjunct marker for further evaluation regarding risk stratification of patients undergoing colonoscopy surveillance [31-34].
Many research efforts were performed to explore the relation between AMACR expression and clinic-pathological features in variable malignancies. Rubin et al. [32] reported correlation between AMACR expression and prostatic carcinoma recurrence and cancer specific death. Xu and Huang [17,35] suggested AMACR as prognostic biomarker for early recurrence and metastasis of hepatocellular carcinoma. Similar results were obtained in gastrointestinal stromal tumours [36], nasopharyngeal carcinoma [37].
Despite these promising data about value of AMACR as diagnostic and prognostic adjunct in surgical pathology, there are limited reports about the relation of AMACR immune-staining to clinic-pathological variables and prognostic parameters of colonic malignancies. In the present study, we analysed immunohistochemical expression of AMACR in normal colonic mucosa, colorectal adenomas, CRC and metastatic CRC to lymph nodes. None of normal colonic mucosae showed positive immunoreactions to AMACR except for occasional positivity in cytoplasm of some surface epithelial cells which were considered as negative staining. AMACR immune-positivity was noted in colorectal adenomas, but no significant correlation was found between its immune-staining and any clinic-pathological variables or degree of dysplasia. Our results are comparable to those of Went et al [37] who did not find any significant correlation between AMACR expression and clinic-pathological features of colorectal adenomas. On the other hand, Lakis [16] and Starter [34] reported that increased immunostaining of AMACR in colorectal adenomas was associated with villous pattern, adenomas larger than 1 cm in diameter, high grade dysplasia and /or carcinoma in situ, whereas adenomas with tubular features, small size and low grade dysplasia showed weak positive staining to AMACR. AMACR was expressed in 53.6% of CRC cases and in 36.8% of metastatic CRC to Lymph nodes. Our result is comparable to other investigators who reported AMACR positivity in 47 to 81% of CRC cases [38-40]. We found significant correlation between AMACR down regulation with the occurrence of lymph node metastasis and distant metastasis, but no association between AMACR immune-staining and clinic-pathological features as age, sex, degree of differentiation, depth of tumour invasion, tumour stage, lymphvascular invasion, and status of surgical resection margins. Our results are contradictory to those of Marx, et al. [39] who reported positive relation between reduced AMACR immunostaining and high tumour grade, stage and occurrence on left colon but did not find any relation to lymph node status or distant metastasis.
Conclusion
AMACR immunostaining is correlated with nodal metastasis and distant metastasis. AMACR is an independent predictor of lymphvascular invasion, positive surgical margin, nodal and distant metastasis. AMACR may serve as biomarker of progression and prognosis of CRC.
Acknowledgement
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH)-King Abdulaziz City for Science and Technology - the Kingdom of Saudi Arabia-award number (11-BIO1524-03). The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support.
References
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108.
- Roynette CE, Calder PC, Dupertuis YM, Pichard C (2004) n-3 polyunsaturated fatty acids and colon cancer prevention. ClinNutr 23: 139-151.
- Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, et al. (2011) Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 6:e20456.
- Theodoratou E, McNeill G, Cetnarskyj R, Farrington SM, Tenesa A, et al. (2007) Dietary fatty acids and colorectal cancer: a case-control study. Am J Epidemiol 166: 181-195.
- zurHausen H(2012): Red meat consumption and cancer: reasons to suspect involvement of bovine infectious factors in colorectal cancer. Int J Cancer, 130:2475-2483.
- Lloyd MD, Darley DJ, Wierzbicki AS, Threadgill MD (2008) Alpha-methylacyl-CoA racemase--an 'obscure' metabolic enzyme takes centre stage. FEBS J 275: 1089-1102.
- Lloyd MD, Yevglevskis M, Lee GL, Wood PJ, Threadgill MD, et al. (2013) α-Methylacyl-CoA racemase (AMACR): metabolic enzyme, drug metabolizer and cancer marker P504S. Prog Lipid Res 52: 220-230.
- Jiang Z, Fanger GR, Woda BA, Banner BF, Algate P, et al. (2003) Expression of alpha-methylacyl-CoA racemase (P504s) in various malignant neoplasms and normal tissues: astudy of 761 cases. Hum Pathol 34: 792-796.
- Nassar A, Amin MB, Sexton DG, Cohen C (2005) Utility of alpha-methylacyl coenzyme A racemase (p504s antibody) as a diagnostic immunohistochemical marker for cancer. ApplImmunohistochemMolMorphol 13: 252-255.
- Ferdinandusse S, Denis S, Clayton PT, Graham A, Rees JE, et al (2000). Mutations in the gene encoding peroxisomal alpha-methylacyl-CoA racemase cause adult-onset sensory motor neuropathy. Nat Genet 24:188-191.
- Ouyang B, Leung YK, Wang V, Chung E, Levin L, et al. (2011).Alpha-Methylacyl-CoA racemase spliced variants and their expression in normal and malignant prostate tissues. Urology 77:249 e1-249e7.
- Wright JL, Neuhouser ML, Lin DW, Kwon EM, Feng Z, et al. (2011) AMACR polymorphisms, dietary intake of red meat and dairy and prostate cancer risk. Prostate 71: 498-506.
- Wright ME, Bowen P, Virtamo J, Albanes D, Gann PH (2012) Estimated phytanic acid intake and prostate cancer risk: a prospective cohort study. Int J Cancer 131: 1396-1406.
- Zha S, Ferdinandusse S, Denis S, Wanders RJ, Ewing CM, et al. (2003) Alpha-methylacyl-CoA racemase as an androgen-independent growth modifier in prostate cancer. Cancer Res 63:7365-7376.
- Jiang Z, Fanger GR, Banner BF, Woda BA, Algate P, et al. (2003) A dietary enzyme: alpha-methylacyl-CoA racemase/P504S is overexpressed in colon carcinoma. Cancer Detect Prev 27:422-426.
- Lakis S, Papamitsou T, Panagiotopoulou C, Kotakidou R, Kotoula V (2010) AMACR is associated with advanced pathologic risk factors in sporadic colorectal adenomas. World J Gastroenterol 16: 2476-2483.
- Xu B, Cai Z, Zeng Y, Chen L, Du X, et al. (2014) α-Methylacyl-CoA racemase (AMACR) serves as a prognostic biomarker for the early recurrence/metastasis of HCC. J ClinPathol 67: 974-979.
- Duyar SS, Yilmaz A, Demirag F, Erdogan Y, Yazici U, et al. (2015) The expression and clinical effects of alpha-methylacyl-CoA racemase (AMACR/ P504S) as an immunohistochemical marker in malign pleural mesothelioma. Turk J Med Sci 45:607-614.
- Aron M, Luthringer DJ, McKenney JK, Hansel DE, Westfall DE, et al. (2013) Utility of a triple antibody cocktail intraurothelial neoplasm-3 (IUN-3-CK20/CD44s/p53) and alpha-methylacyl-CoA racemase (AMACR) in the distinction of urothelial carcinoma in situ (CIS) and reactive urothelialatypia. Am J SurgPathol 37:1815-1823.
- Ollberding NJ, Aschebrook-Kilfoy B, Caces DB, Wright ME, Weisenburger DD, et al. (2013) Phytanic acid and the risk of non-Hodgkin lymphoma. Carcinogenesis 34: 170-175.
- Pramick M, Ziober A, Bing Z (2013) Useful immunohistochemical panel for differentiating clear cell papillary renal cell carcinoma from its mimics. Ann DiagnPathol 17: 437-440.
- Abbas M, Ploch EM, Wehling J, Schipper E, Janciauskiene S, et al. (2014) alpha-Methylacyl-coenzyme A racemase (AMACR, p504s) is a marker to distinguish malignant melanomas from dysplastic nevi and melanocytic nevi. Tumour Biol 35:12015-12020.
- Mroz A, Kiedrowski M, Lewandowski Z (2013) α-Methylacyl-CoA racemase (AMACR) in gastric cancer: correlation with clinicopathologic data and disease-free survival. ApplImmunohistochemMolMorphol 21: 313-317.
- Noske A, Zimmermann AK, Caduff R, Varga Z, Fink D, et al. (2011) Alpha-methylacyl-CoA racemase (AMACR) expression in epithelial ovarian cancer. Virchows Arch 459: 91-97.
- Witkiewicz AK, Varambally S, Shen R, Mehra R, Sabel MS, et al. (2005) Alpha-methylacyl-CoA racemase protein expression is associated with the degree of differentiation in breast cancer using quantitative image analysis. Cancer Epidemiol Biomarkers Prev, 14:1418-1423.
- Edge S, Byrd D, Compton C (2010) AJCC Cancer Staging Handbook, 7th edition edn. New York: Springer.
- Al-Maghrabi J, Gomaa W, Buhmeida A, Qari Y, Al-Qahtani M, et al. (2014) Prognostic significance of VEGFR1/Flt-1 immunoexpression in colorectal carcinoma. Tumour Biol 35: 9045-9051.
- Lin PY, Cheng KL, McGuffin-Cawley JD, Shieu FS, Samia AC, et al. (2012) Detection of Alpha-Methylacyl-CoA Racemase (AMACR), A Biomarker of Prostate Cancer, in Patient Blood Samples Using a Nanoparticle Electrochemical Biosensor. Biosensors (Basel), 2:377-387.
- Shin JH, Bae JH, Lee A, Jung CK, Yim HW, et al. (2010) CK7, CK20, CDX2 and MUC2 Immunohistochemical staining used to distinguish metastatic colorectal carcinoma involving ovary from primary ovarian mucinous adenocarcinoma. Jpn J ClinOncol 40:208-213.
- Fadare O, Parkash V, Gwin K, Hanley KZ, Jarboe EA, et al. (2013) Utility of alpha-methylacyl-coenzyme-A racemase (p504s) immunohistochemistry in distinguishing endometrial clear cell carcinomas from serous and endometrioid carcinomas. Hum Pathol44:2814-2821.
- Dorer R, Odze RD (2006) AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett's esophagus, ulcerative colitis, and Crohn's disease. Am J SurgPathol 30: 871-877.
- Rubin MA, Bismar TA, Andren O, Mucci L, Kim R, et al. (2005) Decreased alpha-methylacyl CoA racemase expression in localized prostate cancer is associated with an increased rate of biochemical recurrence and cancer-specific death. Cancer Epidemiol Biomarkers Prev 14:1424-1432.
- Sonwalkar SA, Rotimi O, Scott N, Verghese E, Dixon M, et al. (2010) A study of indefinite for dysplasia in Barrett's oesophagus: reproducibility of diagnosis, clinical outcomes and predicting progression with AMACR (alpha-methylacyl-CoA-racemase). Histopathology 56:900-907.
- Strater J, Wiesmuller C, Perner S, Kuefer R, Moller P(2008) Alpha-methylacyl-CoA racemase (AMACR) immunohistochemistry in Barrett's and colorectal mucosa: only significant overexpression favours a diagnosis of intraepithelial neoplasia. Histopathology52:399-402.
- Huang X, Zeng Y, Xing X, Zeng J, Gao Y, et al. (2014) Quantitative proteomics analysis of early recurrence/metastasis of huge hepatocellular carcinoma following radical resection. Proteome Sci 12:22.
- Li CF, Chen LT, Lan J, Chou FF, Lin CY, et al. (2014) AMACR amplification and overexpression in primary imatinib-naive gastrointestinal stromal tumors: a driver of cell proliferation indicating adverse prognosis. Oncotarget 5: 11588-11603.
- Went PT, Sauter G, Oberholzer M, Bubendorf L (2006) Abundant expression of AMACR in many distinct tumour types. Pathology 38: 426-432.
- Lin A, Weiser MR, Klimstra DS, Paty PB, Tang LH, et al. (2007) Differential expression of alpha-methylacyl-coenzyme A racemase in colorectal carcinoma bears clinical and pathologic significance. Hum Pathol 38: 850-856.
- Marx A, Simon P, Simon R, Mirlacher M, Izbicki JR, et al. (2008) AMACR expression in colorectal cancer is associated with left-sided tumor localization. Virchows Arch 453:243-248.
- Shi X, Gong E, Wu X (2007) Alpha-methylacyl-CoA racemase/P504S overexpression in colorectal carcinoma is correlated with tumor differentiation. ApplImmunohistochemMolMorphol 15: 175-180.
Citation: Emam E, Gomaa W, Al-Ahwal M, Mushref R, Al-Maghrabi B, et al. (2016) Lack of AMACR Immunostaining is an Independent Predictor of Poor Prognosis in Colorectal Carcinoma. J Clin Exp Pathol 6:279. Doi: 10.4172/2161-0681.1000279
Copyright: © Maghrabi JA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 13201
- [From(publication date): 6-2016 - Apr 19, 2024]
- Breakdown by view type
- HTML page views: 12417
- PDF downloads: 784