The Orthopaedic Consequences of Childhood Meningococcal Septicaemia
Received: 08-Jul-2016 / Accepted Date: 19-Sep-2016 / Published Date: 26-Sep-2016 DOI: 10.4172/2572-2050.1000109
Abstract
Purpose: The aim of this study is to use a defined population of patients with meningococcal septicaemia to calculate the incidence of orthopaedic complications.
Methods: Medical records and radiographs were analysed retrospectively for all patients admitted to the Paediatric Intensive Care Unit (PICU) of the Bristol Royal Hospital for Children from 01/01/2001 to 31/12/2012 with a primary diagnosis of meningococcal septicaemia.
Results: Of the 130 patients with meningococcal septicaemia alive at discharge, 10 developed orthopaedic sequelae, representing an overall incidence in this patient population of 7.7%. 9 patients required an amputation, 8 patients had growth plate abnormalities with 6 having documented angular deformity.
Clinical relevance: This study highlights the underestimation of orthopaedic complications following meningococcal septicaemia. Close follow up of at risk patients should be considered to reduce the potential impact of these debilitating injuries.
Keywords: Amputation; Orthopaedic; Tibia; Ischaemia; Periosteum
Introduction
Meningococcal septicaemia is caused by the Gram-negative diplococcus, Neisseria meningitides. The bacterium is found exclusively in humans, existing as a commensal in the nasopharynx and transmitted by respiratory secretions. Meningoccoal septicaemia is the most severe form of meningococcal infection and is the most common infective cause of death in children [1].
Differing hospital settings, patient populations and disease presentations make generalising mortality rates following meningococcal septicaemia difficult to calculate. There has however been an overall decline in the death rate, with earlier studies reporting a rate of 18-53% [2], while more recent estimates are as low as 5% [3]. Improved treatment protocols and centralisation of paediatric intensive care is thought to have had a major effect [4]. The consequence of the increased survival of meningococcal patients is an increased prevalence of long term complications including hearing loss [5], renal failure [6], neurological impairment [7], behavioral problems [7] and skin complications [8].
There is a significant gap in knowledge regarding the incidence of orthopaedic complications following meningococcal septicaemia. In addition, the relative distribution of these complications has not been adequately described. A number of reports during the 1980’s [9-11] attempted to document the various complications, but there has been no conclusive description of the incidence and variety of orthopaedic involvement. This lack of knowledge is reflected by the absence of relevant information provided by the National Institute for Health and Care Excellence [12].
The primary aim of this study is to use a defined population as a denominator to enable correct identification of the incidence of orthopaedic complications following meningococcal septicaemia. The secondary aim is to identify patients who are at an increased risk of developing orthopaedic sequelae in order to provide surveillance and earlier intervention.
Methods
Patient selection
All the patients admitted to the Paediatric Intensive Care Unit (PICU) of our institution between 01/01/2001 to 31/12/2012 with a primary diagnosis of ‘Bacterial meningitis’ or ‘Meningococcal septicaemia’ was identified. Those diagnosed with meningococcal septicaemia were used as the initial cohort. Children without a definite diagnosis of meningococcal septicaemia were excluded. Retrospective analysis was performed on all clinic letters, correspondences and radiographs from the day of PICU admission to the time of writing. Information was gathered regarding the age, sex, length of PICU admission and mortality of these patients. Orthopaedic complications were defined as any amputation (spontaneous or surgical) or growth plate abnormality (leading to shortening or angular deformity) and any skin changes were also recorded.
Statistical assessment
Data was collated using Microsoft Excel (Microsoft Corporation, Seattle, Washington) and analysed using SPSS statistical software (IBM SPSS 19). Data is displayed as group means. Following a Kolmogorov-Smirnov test, groups were compared using the Mann-Whitney U test. P values <0.05 were considered statistically significant. Risk ratios were calculated, and the 95% confidence intervals (CI) stated.
Results
PICU patients
285 patients met the initial inclusion criteria and of these, 147 (51.6%) had some form of bacterial meningitis and 138 (48.4%) had a primary diagnosis of meningococcal septicaemia (Table 1). 8 patients with meningococcal septicaemia died, representing a mortality rate of 5.8%. Of the remaining 130 patients, none died of any cause following PICU admission.
| Total number of patients | 138 |
| Total alive at discharge | 130 |
| M:F ratio | 06:05 |
| Average age at PICU admission (years) | 3.6 |
| Average length of PICU admission (days) | 4 |
Table 1: Patients with meningococcal septicaemia admitted to the Paediatric Intensive Care Unit.
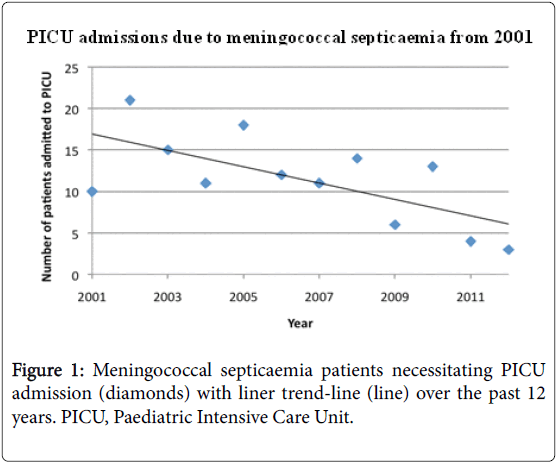
The varying incidence of meningococcal septicaemia necessitating PICU admission between 2001 and 2012 is shown in Figure 1. There is a gradual decline in the overall admission rate for meningococcal septicaemia from 10 and 21 cases in 2001 and 2002 respectively to 4 and 3 cases in 2011 and 2012.
Orthopaedic complications
Of the 130 patients who were alive at PICU discharge, 10 went on to develop orthopaedic sequelae, representing an overall incidence in this patient population of 7.7% (Table 2). A greater proportion of boys developed orthopaedic complications, although this was not statistically significant (Risk ratio 3.12; 95% CI 0.69–14.14). Those who developed complications were admitted to PICU at a significantly younger age (P<0.05) and for a significantly longer time (P<0.001).
| Orthopaedicsequelae | No orthopaedic sequelae | Risk ratio (95% CI)/P value | |
|---|---|---|---|
| Total number | 10 | 120 | n/a |
| M:F ratio | 04:01 | 06:05 | 3.12 (0.69–14.14) |
| Average age at PICU admission (years) | 1.5 | 3.9 | <0.05 |
| Average length of PICU admission (days) | 15.2 | 4 | <0.001 |
| Skin involvement (%) | 100 | 0.8 | <0.001 |
Table 2: Comparison of those who did and those who did not develop.
All those with orthopaedic complications had noticeable skin involvement, as compared to only a single case in those who were unaffected.
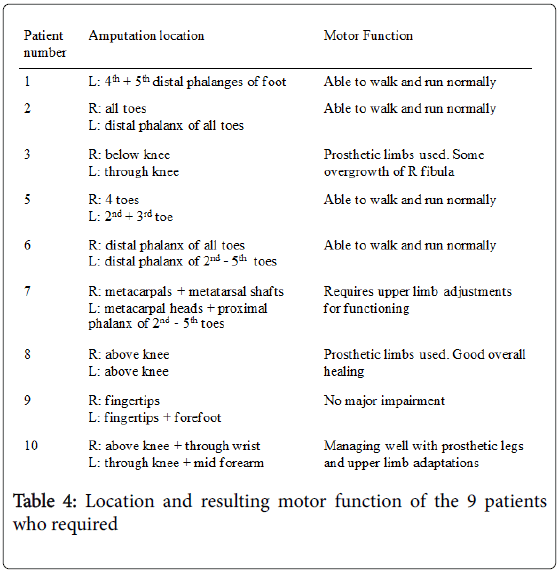
Ten patients developed some form of orthopaedic sequelae (Table 3). All patients, bar one who later presented with leg length discrepancy, required an amputation as treatment for the acute illness.
| Case | Gender | Age at sepsis (months) | Length of PICU admission (days) | Acute amputation | GPA | Skin involvement |
|---|---|---|---|---|---|---|
| 1 | F | 8.5 | 7 | Y | Y | Y |
| 2 | M | 17.3 | 5 | Y | Y | Y |
| 3 | M | 7.8 | 21 | Y | Y | Y |
| 4 | M | 33.2 | 18 | N | Y | Y |
| 5 | M | 47.6 | 4 | Y | Y | Y |
| 6 | F | 13.4 | 23 | Y | Y | Y |
| 7 | M | 8.8 | 16 | Y | Y | Y |
| 8 | M | 16.5 | 28 | Y | N | Y |
| 9 | M | 11.1 | 10 | Y | Y | Y |
| 10 | M | 12.2 | 20 | Y | N | Y |
Table 3: Overview of the 10 patients who developed orthopaedic complications following meningococcal septicaemia. PICU, Paediatric Intensive Care Unit; GPA, Growth plate abnormalityOverview of the 10 patients who developed orthopaedic complications following meningococcal septicaemia. PICU, Paediatric Intensive Care Unit; GPA, Growth plate abnormality.
Growth plate abnormalities were seen in 8 patients during followup. 7 patients had both an amputation and growth plate abnormality. The only common finding across all patients was the presence of skin changes following meningococcal septicaemia.
All patients requiring an amputation during their PICU admission were subsequently monitored closely as outpatients. Consequent growth plate arrest was therefore noted by the orthopaedic team. Patient 4 did not require an amputation and was not under orthopaedic monitoring. He was referred to the orthopaedic team from his general practitioner due to an apparent leg length discrepancy. During subsequent care, further growth plate abnormalities were detected. The interval between PICU admission and orthopaedic referral of Patient 4 was 3.6 years.
Amputations
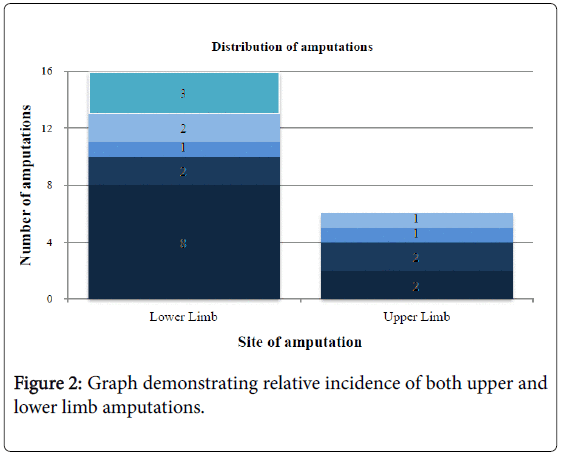
Table 4 demonstrates there was no predilection for right or left involvement, with 8/9 patients having bilateral involvement to a similar degree. However, Figure 2 shows that 16/22 (72.7%) of the limbs involved were lower limbs.
Revision of amputation was required in two patients. Patient 3 initially had a spontaneous right midtarsal amputation. Scarred and unstable skin necessitated later revision to a mid-calf amputation. Patient 10 required revision to a higher level on the right leg due to bone overgrowth.
Growth plate abnormalities
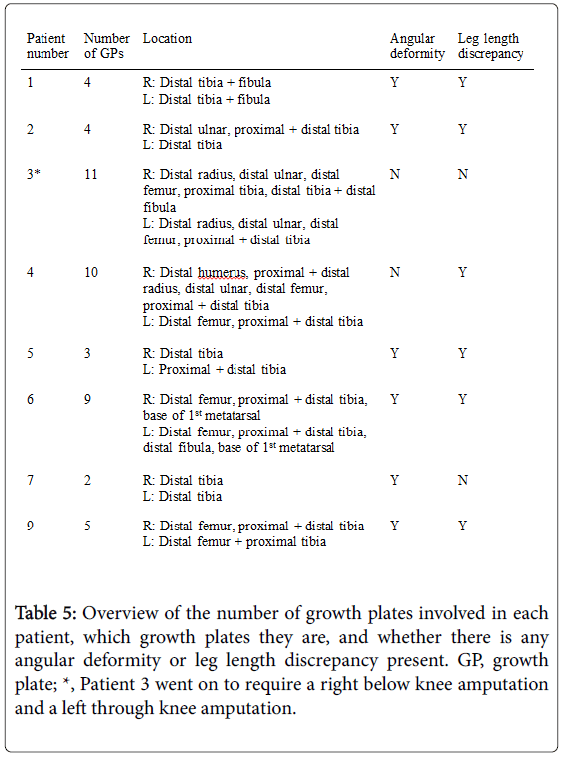
Eight patients had multiple growth plate abnormalities. Table 5 shows that there were a total of 48 abnormal growth plates in the 8 patients.
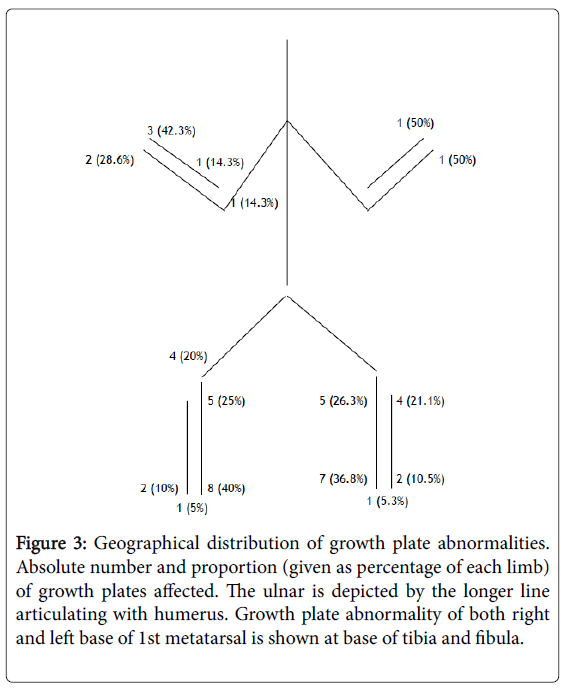
Figure 3 demonstrates there was no significant difference in terms of side, with 27 (56.3%) growth plate abnormalities on the right, and 21 (43.8%) on the left. There was a tendency for lower limb involvement however, with 39 (81.3%) of the growth plates affected in the lower limb, compared to only 9 (18.8%) in the upper limb.
Figure 3: Geographical distribution of growth plate abnormalities. Absolute number and proportion (given as percentage of each limb) of growth plates affected. The ulnar is depicted by the longer line articulating with humerus. Growth plate abnormality of both right and left base of 1st metatarsal is shown at base of tibia and fibula.
The most common growth plate affected in the right (40%) and left (36.8%) lower limb was the distal tibia. Bilaterally, the distal tibia represents 15/39 (38.5%) of the growth plate abnormalities in the leg. Neither proximal femur nor proximal fibula was involved in any patient.
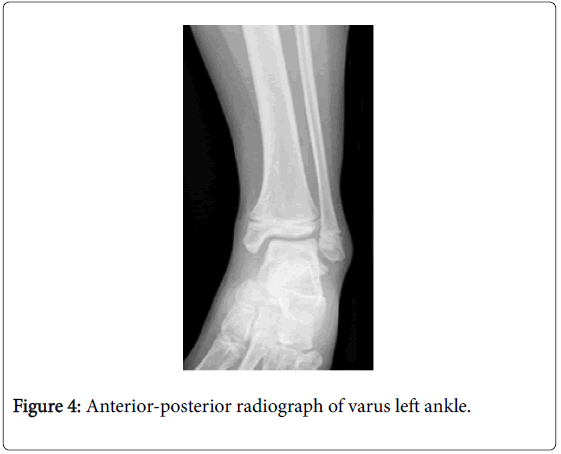
Angular deformity was a common consequence of partial growth plate arrest. Of the 6 patients with angular deformity, 10 ankles were identified as having a varus malalignment. Figure 4 demonstrates fusion across the central portion of the left distal tibial physis. The distal fibula is affected to a much lesser extent than the distal tibia resulting in relative overgrowth of the fibula contributing to the varus deformity.
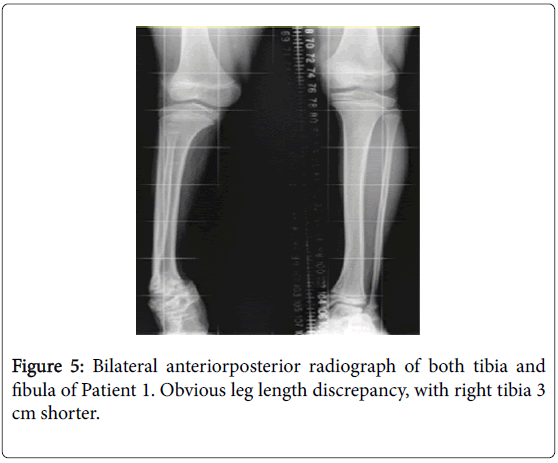
There was a leg length discrepancy documented in 6 patients with growth plate abnormalities. The tibia was the most commonly affected bone (Figure 5), with all discrepancies having some contribution from the tibia.
The degree of tibial shortening varied from 1.5 cm to 3 cm. Patients 4 and 9 had shortening of one femur, with the difference documented as 1 cm in Patient 9.
Skin involvement
All 10 patients had documented skin changes (Table 3). Skin scarring was the most common finding. All but patient 9 had noticeable cutaneous scarring superficially to a damaged growth plate. The most commonly reported scar was on the lower leg lying medially. Other complications included 3 fasciotomies for compartment syndrome, an acute complication of meningococcal septicaemia. All 10 patients had experienced skin necrosis, most commonly in the distal toes, or purpura fulminans during their admission.
Of the 120 patients admitted with meningococcal septicaemia, but no reported orthopaedic complications, none had any reported acute skin changes. Additionally, none had been referred back to the orthopaedic team as a result of abnormally looking skin.
Discussion
Previous studies investigating the orthopaedic consequences of childhood meningococcal septicaemia have considered an unselected population [13-23]. As such, they are unable to give accurate demographic data. The methodology applied here overcomes the limitations of using data derived from multiple centres [15] or insufficient patient numbers [16]. The information gathered by this study gives clear, reliable data on the incidence and distribution of orthopaedic complications following meningococcal septicaemia.
Meningococcal septicaemia is a destructive disease that primarily affects children. Data from Melbourne, Australia between 1985 and 2002 on the management and outcomes of 143 patients with meningococcal sepsis noted a mortality rate of just under 15% [13]. Their study predated the introduction of the meningococcal C vaccine to the National Immunisation Program in 2003. In contrast, meningococcal C vaccination was introduced in the UK’s national vaccination program in 1999 and all of the patients in this study should have been immunised. It has previously been confirmed that serogroup C is associated with a higher mortality rate than serogroup B (14% vs 7%) [14], and may explain in part the improved survival in this study.
This study highlights that patients who develop orthopaedic complications following meningococcal septicaemia are admitted to PICU an average of 2.4 years younger than those who do not, supporting previous studies [15-17]. This is likely to be a result of agedependent vulnerability of bone vasculature, as well as the stage of bone development and maturity [18]. Furthermore, those patients developing orthopaedic sequelae remained in PICU for an average of 11.2 days longer. To our knowledge, this is the first study that reports a predilection for orthopaedic sequelae in boys with an increased risk ratio of 3.12 which was not statistically significant. Despite lacking clinical markers for disease severity, infections necessitating limb amputation as initial management is suggestive of later developing growth plate abnormalities. All those who had initial amputations had later growth plate problems. Only one patient developed leg length discrepancy without a previous amputation.
Of those patients who survived the initial disease, 6.9% required an amputation at some level, concurring with 8% of survivors in Buysse et al. [17]. Erickson and De Wals [19] noted that the most common amputation in their study (38.5% of patients) was of multiple toes, which was replicated by this study (55.6% of patients).
Our study identified 8 patients with documented growth plate abnormalities following meningococcal septicaemia, representing an overall incidence of 6.2%, double that predicted by NICE [12]. Their initial underestimation is perhaps due to a lack of available published data, as well as a misunderstanding of the chronic and often subtle nature of growth plate disturbance.
The distal tibia is the most commonly affected growth plate, with 15/39 (38.5%) abnormalities located there. Distal tibial physeal arrest has previously been reported as a consequence of meningococcal septicaemia in children [13,15,20-23]. It has been suggested that the distal tibial physis is usually incompletely damaged, with the medial tibial physis most often involved, leading to partial growth arrest and asymmetrical development [21]. The deformity progresses rapidly due to the distal fibula rarely being affected [23].
In this study, 6 patients (4.6%) had documented limb length discrepancy in agreement with the incidence reported by Buysse et al. (6%) [17]. Our study was able to confirm that the tibia was involved in all cases with a resultant leg length discrepancy, with the femur contributing in two cases. There is a paucity of information in the literature and previous studies lack details of individual bone involvement.
In this series, all patients with growth plate damage had scarring of the adjacent skin, in keeping with previous studies [21]. Skin involvement in our study was exclusive to those presenting with other orthopaedic complications, contrasting with evidence presented by Buysse et al. [17] who report an overall incidence of skin scarring of 48%. The difference may be a reflection of varying data collection methods. Buysse et al. [17] invited patients to a follow-up clinic 4-16 years post discharge, and were therefore more likely to note minor skin scarring. Patients with ‘barely visible’ scars, that were reported by Buysse et al. [17], are less likely to present to healthcare practitioners following discharge and may therefore be underestimated in our study. However, this report does highlight cutaneous scarring as a marker of deep soft tissue injury. As the zone of injury extends it involves the growth plate leading to subsequent growth plate abnormality, angular deformities and leg length differences.
The pathological process that leads to amputations and growth plate damage is likely to be multifactorial. In the early 1960’s Trueta and Amato [24] noted changes in the growth plate as a result of experimentally induced ischemia. More recent human studies suggested that there is a direct injury to the physis as a result of endotoxin-induced microvascular damage leading to ischaemia [25,26]. An indirect mechanism may also be associated with full thickness involvement of the skin and underlying tissue, resulting in an interruption to the angiosomal blood supply [27]. Damage to either true anastomotic connections or reduced caliber choke vessels linking adjacent vascular territories may represent a pathological mechanism. In comparison to the posterior and lateral compartments of the lower leg, where at least two source vessels exist, the anterior and medial aspects may be at increased risk. This is due to the sparseness of choke vessels across the boundaries of the anterior compartment, and the particularly susceptible periosteum and cutaneous vessels that connect the medial subcutaneous area with the anterior and posterior tibial arteries.
Limitations
This study has gathered significant information regarding the known and clinically obvious consequences of meningococcal septicaemia. However, we are unable to confidently exclude the possibility that those patients classified as having ‘No orthopaedic complications’ may have unrecognized orthopaedic morbidity. The methodology used here has the potential to underestimate subtle and evolving injuries.
Knowledge of specific organisms responsible for causing septicaemia in both those that do, and those that do not develop complications was not available. Future work should attempt to identify organisms known to increase the risk of orthopaedic complications in order to alert healthcare professionals to the need for long term surveillance.
Conclusion And Clinical Implications
This study aimed to identify the incidence of orthopaedic complications following meningococcal septicaemia. Using a defined population as a denominator, we have demonstrated an overall incidence of 7.7%. Amputations of the extremities were the most common acute orthopaedic complication. Angular and axial limb deformity developed gradually and skin involvement was seen in all patients with orthopaedic sequelae.
Future research should focus on detecting at risk children in order to ensure close follow up and early identification of deformities which may lead to improvements in orthopaedic outcomes.
References
- Paize F, Playfor SD (2007) Improvements in the outcome of children with meningococcal disease. Crit Care 11: 172.
- Kirsch EA, Barton RP, Kitchen L, Giroir BP (1996) Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J 15: 967-978.
- Pollard AJ1, Britto J, Nadel S, DeMunter C, Habibi P, et al. (1999) Emergency management of meningococcal disease. Arch Dis Child 80: 290-296.
- Maat M, Buysse CM, Emonts M, Spanjaard L, Joosten KF, et al. (2007) Improved survival of children with sepsis and purpura: effects of age, gender, and era. Crit Care 11: R112.
- Dawson JA, Wardle R (1990) Detection and prevalence of hearing loss in a cohort of children following serogroup B, meningococcal infection 1983-1987. Public Health 104: 99-102.
- Slack R, Hawkins KC, Gilhooley L, Addison GM, Lewis MA, et al. (2005) Long-term outcome of meningococcal sepsis-associated acute renal failure. PediatrCrit Care Med 6: 477-479.
- Buysse CM, Vermunt LC, Raat H, Hazelzet JA, Hop WC, et al. (2010) Surviving meningococcal septic shock in childhood: long-term overall outcome and the effect on health-related quality of life. Crit Care 14: R124.
- Join-Lambert O, Lecuyer H, Miller F, Lelievre L, Jamet A, et al. (2013) Meningococcal interaction to microvasculature triggers the tissular lesions of purpurafulminans. J Infect Dis 208: 1590-1597.
- Fernandez F, Pueyo I, Jimenez JR, Vigil E, Guzman A (1981) Epiphysiometaphyseal changes in children after severe meningococcic sepsis. Am J Roentgenol 136: 1236-1238.
- Barre P, Thompson GH, Morrison SC (1985) Late skeletal deformities following meningococcal sepsis and disseminated intravascular coagulation. J Pediat Orthop 5: 584-588
- Duncan JS, Ramsey LE (1984) Widespread bone infarction complicating meningococcal septicaemia and disseminated intravascular coagulation. British Med J 288: 111-112.
- National Collaborating Centre for Women's and Children's Health (UK) (2010) Bacterial meningitis and meningococcal septicaemia. Management of bacterial meningitis and meningococcal septicaemia in children and young people younger than 16 years in primary and secondary care. London: RCOG Press.
- Bache CE, Torode IP (2006) Orthopaedicsequelae of meningococcal septicemia. J PediatrOrthop 26: 135-139.
- Steven N, Wood M (1995) The clinical spectrum of meningococcal disease. Chichester: John Wiley & Sons.
- Belthur MV1, Bradish CF, Gibbons PJ (2005) Late orthopaedicsequelae following meningococcal septicaemia. A multicentre study. J Bone Joint Surg Br 87: 236-240.
- Nectoux E1, Mezel A, Raux S, Fron D, Maillet M, et al. (2010) Meningococcal purpurafulminans in children: I. Initial orthopedic management. J Child Orthop 4: 401-407.
- Buysse CM, Oranje AP, Zuidema E, Hazelzet JA, Hop WC, et al. (2009) Long-term skin scarring and orthopaedicsequelae in survivors of meningococcal septic shock. Arch Dis Child 94: 381-386.
- Campbell WN, Joshi M, Sileo D (1997) Osteonecrosis following meningococcemia and disseminated intravascular coagulation in an adult: case report and review. Clin Infect Dis 24: 452-455.
- Erickson L1, De Wals P (1998) Complications and sequelae of meningococcal disease in Quebec, Canada, 1990-1994. Clin Infect Dis 26: 1159-1164.
- Canavese F, Krajbich JI, LaFleur BJ (2010) Orthopaedicsequelae of childhood meningococcemia: management considerations and outcome. J Bone Joint Surg Am 92: 2196-2203.
- Monsell FP, Barnes JR, Kirubanandan R, McBride AM (2011) Distal tibialphyseal arrest after meningococcal septicaemia: management and outcome in seven ankles. J Bone Joint Surg Br 93: 839-843.
- O'Sullivan ME1, Fogarty EE (1990) Distal tibialphyseal arrest: a complication of meningococcal septicemia. J PediatrOrthop 10: 549-550.
- Monsell FP, McBride AR, Barnes JR, Kirubanandan R (2011) Angular deformity of the ankle with sparing of the distal fibula following meningococcal septicaemia: a case series involving 14 ankles in ten children. J Bone Joint Surg Br 93: 1131-1133
- Trueta J, Amato VP (1960) The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. J Bone Joint Surg Br 42-42B: 571-87.
- Grogan DP1, Love SM, Ogden JA, Millar EA, Johnson LO (1989) Chondro-osseous growth abnormalities after meningococcemia. A clinical and histopathological study. J Bone Joint Surg Am 71: 920-928.
- Nogi J1 (1989) Physeal arrest in purpurafulminans. A report of three cases. J Bone Joint Surg Am 71: 929-931.
- Taylor GI1, Pan WR (1998) Angiosomes of the leg: anatomic study and clinical implications. PlastReconstrSurg 102: 599-616.
Citation: Edwards TA, Bowen L, Bintcliffe F, Aird J, Monsell F (2016) The Orthopaedic Consequences of Childhood Meningococcal Septicaemia. J Meningitis 1:109. Doi: 10.4172/2572-2050.1000109
Copyright: ©2016 Edwards TA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 18088
- [From(publication date): 9-2016 - Apr 24, 2024]
- Breakdown by view type
- HTML page views: 17108
- PDF downloads: 980