 |
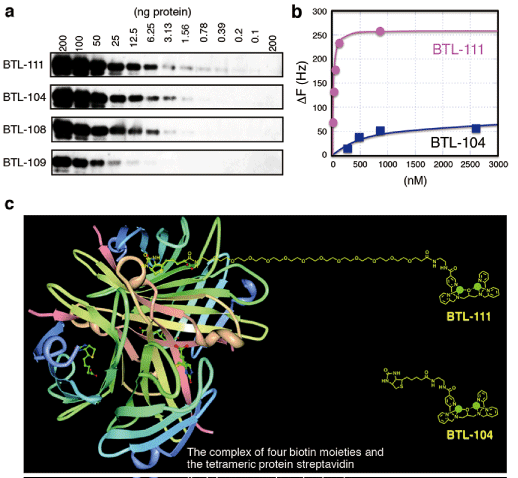
| Figure 3: Development of a novel phosphate-affinity probe. (a) Western blotting analysis of β-casein (200–0.1 ng) and dephosphorylated β-casein (200 ng, rightmost lane) by using complexes of various Phos-tag Biotin derivatives and HRR–SA. All the ECL images were obtained by using Lumigen TMA-6 (as an ECL substrate reagent, Lumigen, Southfield, MI, USA) and the LAS 3000 image analyzer. (b) QCM measurements of binding of β-casein to the surface of phosphate-affinity sensor chips coated with BTL-111 or BTL-104 by using an Affinix QNμ (Initium, Tokyo, Japan). (c) Superimposed images of the complex of four biotin moieties, the tetrameric protein streptavidin, and the newly synthesized derivative BTL-111. For reference, the structure of the derivative BTL-104 is also shown. The introduction of the long hydrophilic spacer [dodeca(ethylene glycol)] increases the flexibility of the phosphatebinding moiety of Phos-tag, resulting in a greater sensitivity in the detection of phosphoproteins and phosphopeptides. [Reprinted with permission from Ref. 28 ©(2012) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim]. |