 |
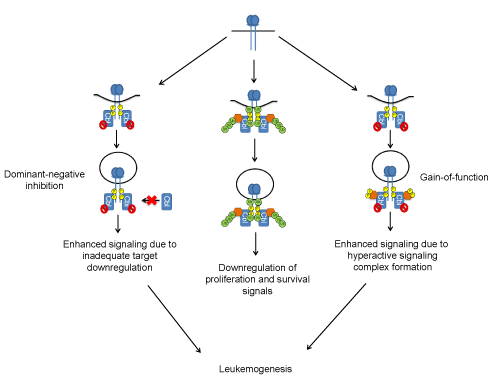
| Figure 3: Potential mechanisms of mutant Cbl-induced leukemogenesis. (left) Mutant Cbl proteins retain the ability to bind to activated protein tyrosine kinases (shown is a generic receptor tyrosine kinase), but cannot promote their ubiquitination. Mutant Cbl (indicated by the red “no” symbol; typically expressed in cancers from duplicated mutant alleles) interacts with activated target tyrosine kinases in competition with remaining wild-type Cbl and/or Cbl-b (dominant-negative inhibition); the ineffective degradation and downregulation of activated protein tyrosine kinases results in enhanced signaling to promote leukemogenesis. (right) Mutant Cbl proteins bound to activated protein tyrosine kinases juxtapose downstream signaling effectors (that interact with Cbl mutants via intact C-terminal regions) to activated tyrosine kinases. These stabilized and hyperactive macromolecular signaling complexes enhance signaling (gain of function) through the downstream signaling pathways that are negatively regulated by WT Cbl under physiological conditions and possibly by novel pathways. (middle) These mechanisms contrast with the normal case, whereby Cbl binds to activated protein tyrosine kinases and also downstream signaling effectors, ubiquitinating both and resulting in downregulation of signaling. |