 |
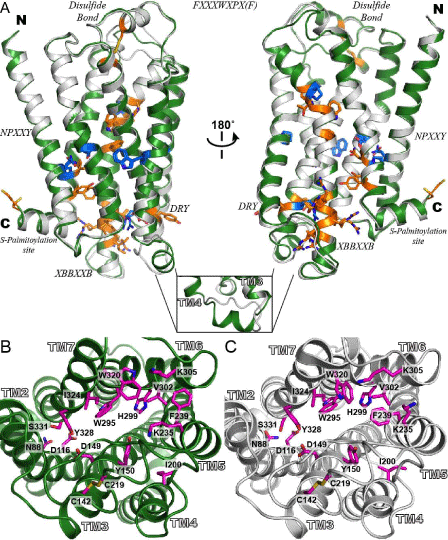
| Figure 2: Alignment and comparison of representative models of hMOP-R. (A) the representative models from the four-template ensemble (4T-hMOPR) and two-template ensemble (2T-hMOP-R) are color in green and white, respectively. The most conserved residues at each of the transmembrane helices are depicted as blue sticks. The highly conserved motifs in the rhodopsin- like GPCR family are depicted as orange sticks; disulfide bond between TM3 and EC2, DRY in TM3, XBBXXB in IC3 (where B represents a basic amino acid and X represents a non-basic residue), FXXXWXPX[F] in TM6, the NPXXY in TM7 and the C-terminal cys palmitoylation site. The inset at the bottom shows one of the most significant differences between the two models. The IC2 loop that connects helices TM3 and TM4 in the 4T-hMOPR model, forms a helical motif inherit from the β1 adrenergic receptor and the A2A adenosine receptor templates. (B) Top views of the binding site for the 4T-hMOP-R and 2T-hMOP-R models with a set of the relevant residues involved in ligand interaction based on mutagenesis studies. Side chain residues are colored in magenta in both cases. This view of the hMOP-R from the extracellular side of the membrane shows the counterclockwise arrangement of the TM helices. |