 |
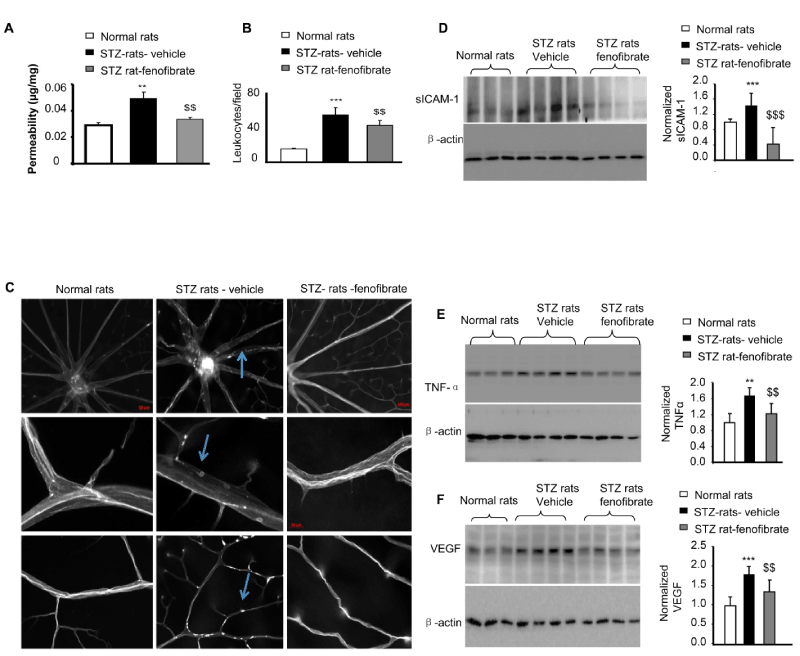
| Figure 3: Topical fenofibrate ameliorates retinal inflammation and reduces retinal vascular leakage in type 1 diabetic animals. STZ-induced diabetic rats were topically administered fenofibrate (15 μl/eye, 3 times/day for 4 weeks) 6 weeks after diabetes onset, and an identical volume of the vehicle served as the control. (A) Retinal vascular leakage was quantified using a vascular permeability assay (mean ± SD, n =5; **P <0.01 STZ vehicle vs. nondiabetic rats (NDM); $$P <0.01 STZ-fenofibrate vs. STZ-vehicle). (B,C) The retinal vascular endothelium and adherent leukocytes were stained using FITC-conjugated Con-A following the removal of circulating leukocytes. The retinas were flat mounted, and adherent leukocytes were visualized using fluorescence microscopy. (B) Leukocyte quantification indicated that topical fenofibrate significantly reduced adherent leukocytes in the retinal vasculature from diabetic rats (mean ± SD, n =7, *** P <0.001 STZ vehicle vs. nondiabetic rats (NDM); $$ P <0.01 STZ-fenofibrate vs. STZ-vehicle). (C) Representative images of retinal flat mounts from nondiabetic rats, STZ-induced diabetic rats treated with vehicle, and diabetic rats treated with fenofibrate eyedrops (30 μl/eye, 3 times/day for 4 weeks) are shown. Scale bar, 100 μm in the first row panel, 50 μm in the second and third row panels. (D-F) Western blot analysis of retinal soluble form ICAM-1 (sICAM-1), TNF-α, and VEGF levels in equivalent amounts (50 μg) of retinal proteins. Each lane represents an individual rat (mean ± SD, n =3 or 4; *STZ vehicle vs. non-diabetic rats (NDM); $STZ-fenofibrate vs. STZ-vehicle; * or $P <0.05; ** or $$P <0.01; *** or $$$P <0.001). |