| Sorafenib: Drug Summary | |
| Name | Sorafenib |
| Chemical formula | C21H16CIF3N4O3 |
| Chemical structure | 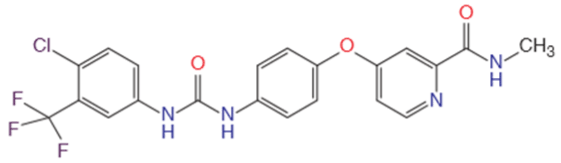 |
| Administration route | Oral |
| Dosage | 400 mg b.i.d. |
| Indication | 2005: FDA approval for advanced renal cell carcinoma. 2006: Marketing authorization given by the European Commission for renal cell carcinoma. 2007: Marketing authorization given by the European Commission for hepatocellular carcinoma; FDA approval for hepatocellular carcinoma. |