
| S.No | R | MIC50a | PIC50 | MIC50b | PIC50 | MIC50c | PIC50 |
| 1 | 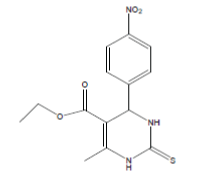 |
35 | 4.4559 | 30 | 4.5228 | 30 | 4.5228 |
| 2 | 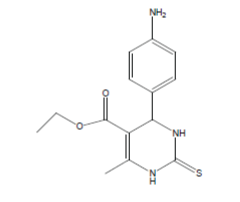 |
40 | 4.3979 | 40 | 4.3979 | 55 | 4.2596 |
| 3 | 2-F | 10 | 5.0132 | 10 | 5.0132 | 10 | 5.0132 |
| 4 | 2-Cl | 10 | 5.0132 | 10 | 5.0132 | 10 | 5.0132 |
| 5 | 2-CF3 | 25 | 4.6020 | 15 | 4.8239 | 25 | 4.6020 |
| 6 | 2-OCF3 | 30 | 4.5228 | 25 | 4.6020 | 15 | 4.8239 |
| 7 | 2-OC6H5 | 40 | 4.3979 | 35 | 4.4559 | 40 | 4.3979 |
| 8 | 2-F, 6-CH3 | 60 | 4.2218 | 30 | 4.5228 | 50 | 4.3010 |
| 9 | 2-F, 6-CF3 | 55 | 4.2596 | 40 | 4.3979 | 55 | 4.2596 |
| 10 | 2-Cl, 6-CH3 | 40 | 4.3979 | 55 | 4.2596 | 40 | 4.3979 |
| 11 | 2-Cl, 6-CF3 | 40 | 4.3979 | 60 | 4.2218 | 60 | 4.2218 |
| 12 | 2-Cl, 5-CF3 | 55 | 4.2596 | 80 | 4.0969 | 60 | 4.2218 |
| 13 | 2-Cl, 4-CF3 | 35 | 4.4559 | 25 | 4.6020 | 35 | 4.4559 |
| 14 | 2-Cl, 6-F | 65 | 4.1870 | 85 | 4.0705 | 40 | 4.3979 |
| 15 | 3-CF3 | 40 | 4.3979 | 20 | 4.6989 | 25 | 4.6020 |
| 16 | 3-Cl, 4-F | 60 | 4.2218 | 85 | 4.0705 | 80 | 4.0969 |
| 17 | 3,5-F | 90 | 4.0457 | - | - | 95 | 4.0222 |
| 18 | 3,4-CH3 | 90 | 4.0457 | - | - | 95 | 4.0222 |
| 19 | 4-F, 3-CH3 | 65 | 4.1870 | - | - | 80 | 4.0969 |
| 20 | 4-isopropyl | 85 | 4.0705 | 55 | 4.2596 | 90 | 4.0457 |
| 21 | 4-butyl | - | - | 95 | 4.0222 | - | |
| 22 | 4-CF3 | 20 | 4.6989 | 10 | 5.0132 | 15 | 4.8239 |
| 23 | 4-OCH3 | 15 | 4.8239 | 15 | 4.8239 | 10 | 5.0132 |
aStaphylococcus aureus; b Escherichia coli;cCandida albicans