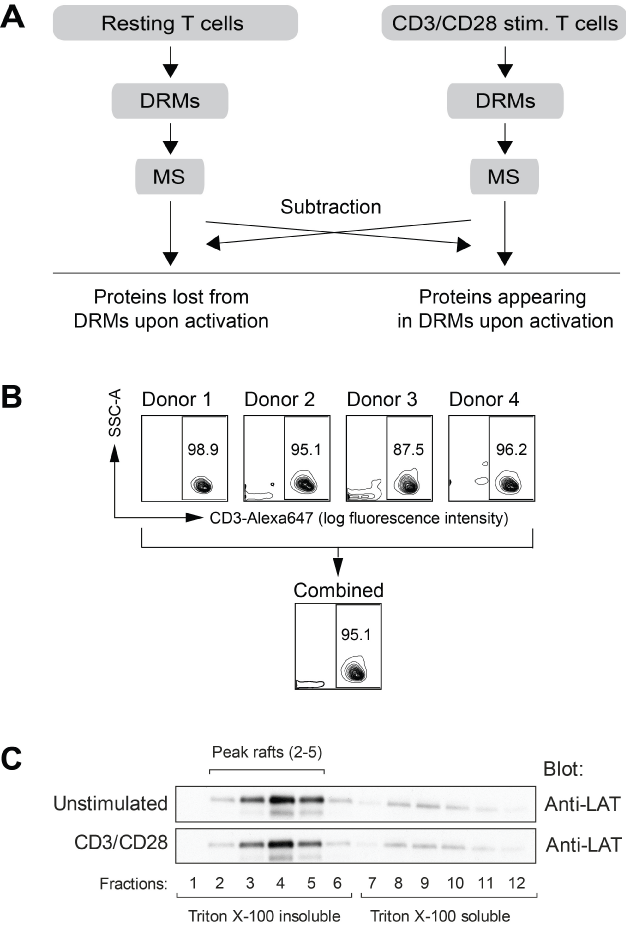
(A) Primary CD3+ T cells from four healthy donors were pooled together and left resting or stimulated (stim.) with anti-CD3 and anti-CD28 for two minutes followed by cross-ligation with F(ab’)2-fragments for three minutes. After lysis on ice using 0.7% Triton X-100, detergent-resistant membranes (DRMs) were isolated by sucrose gradient ultracentrifugation at 4°C. The ultra-light fractions (fractions 2-5) were pooled and lipids removed by chloroformmethanol extraction. Precipitated proteins were subsequently resolved in sample buffer and separated by SDS-PAGE gel electrophoresis. Whole gel lanes were digested with trypsin and analyzed by Nano-LC/LTQ-Orbitrap MS. After a qualitative mass spectrometric analysis, a set of proteins found to be present solely in either DRMs of resting T cells or in DRMS of CD3/CD28 costimulated T cells was identified. (B) One representative experiment using flow cytometry, showing the purity of primary T cells isolated from each of the four blood donors and after pooling of the four populations. (C) Isolation of DRMs (fractions 2-5) was verified by the content of the transmembrane protein LAT (constitutively associated with DRMs).