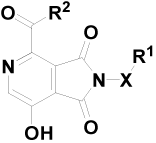
| Entry | Comp. | X | R1 | R2 | EC50b (μM) | CC50c (μM) | TId | |
| 1 | 5a | CH2 | Ph | OEt | 63.37 | 234.02 | 3.69 | |
| 2 | 5b | CH2 | 2-FPh | OEt | 252.56 | >581.40 | >2.30 | |
| 3 | 5c | CH2 | 4-FPh | OEt | 88.89 | 323.32 | 3.64 | |
| 4 | 5d | CH2 | 2-OMePh | OEt | 57.58 | 395.39 | 6.87 | |
| 5 | 5e | CH2 | 3-OMePh | OEt | 213.17 | >561.80 | >2.64 | |
| 6 | 5f | CH2 | 4-OMePh | OEt | 69.18 | 243.69 | 3.52 | |
| 7 | 5g | CH2 | 3,4-OMePh | OEt | 288.91 | >518.13 | >1.79 | |
| 8 | 5h | CH2 | naphthalen-1-yl | OEt | 48.32 | >531.91 | >11.01 | |
| 9 | 5i | CH(CH3) | Ph | OEt | 68.79 | 320.09 | 4.65 | |
| 10 | 6a | NH | 4-FPh | OEt | 32.61 | 213.01 | 6.53 | |
| 11 | 6b | NH | 4-ClPh | OEt | 14.59 | 135.46 | 9.28 | |
| 12 | 7a | CH2CH2 | Ph | OEt | 55.91 | 376.18 | 6.73 | |
| 13 | 7b | CH2CH2 | 2-FPh | OEt | 44.83 | 148.80 | 3.32 | |
| 14 | 7c | CH2CH2 | 3-FPh | OEt | 52.26 | 176.42 | 3.38 | |
| 15 | 7d | CH2CH2 | 4-FPh | OEt | 1.65 | 13.16 | 7.98 | |
| 16 | 7e | CH2CH2 | 4-ClPh | OEt | 15.16 | 145.43 | 9.59 | |
| 17 | 7f | CH2CH2 | 4-MePh | OEt | 2.34 | 15.45 | 6.60 | |
| 18 | 7g | CH2CH2 | 4-SO2NH2 | OEt | 136.42 | 173.46 | 1.27 | |
| 19 | 7h | CH2CH2 | 4-OMePh | OEt | 163.92 | 232.97 | 1.42 | |
| 20 | 7i | CH2CH2 | 3,4-OMePh | OEt | 9.15 | 33.52 | 3.66 | |
| 21 | 7j | CH2CH2 | indol-3-yl | OEt | 9.37 | 26.73 | 2.85 | |
| 22 | 8a | CH2CH2 | 4-MePh | NH(4-FBn) | 40.37 | 164.60 | 4.08 | |
| 23 | 8b | CH2CH2 | 4-MePh | NH(2-OMeBn) | 32.70 | 215.82 | 6.60 | |
| 24 | 8c | CH2CH2 | 4-MePh | NHCH2CH(CH2CH2) | 36.41 | 306.17 | 8.41 | |
| 25 | 8d | CH2CH2 | 4-FPh | NH(4-FBn) | 5.70 | 30.76 | 5.40 | |
| 26 | 8e | CH2CH2 | 4-FPh | NH(2-OMeBn) | 63.01 | 224.25 | 3.56 | |
| 27 | 8f | CH2CH2 | 3,4-OMePh | NH(2-OMeBn) | 149.25 | 217.70 | 1.46 | |
| 28 | 9a | CH2CO | 4-BrPh | OEt | 54.27 | >464.04 | >8.55 | |
| 29 | 9b | CH2CH(CH3) | Ph | OEt | 25.14 | 146.10 | 5.81 | |
| 30 | 9c | CH2CH(Ph) | Ph | OEt | 3.10 | 25.22 | 8.14 | |
| 31 | 9d | CH(COOMe)CH2 | Ph | OEt | 185.55 | 346.88 | 1.87 | |
| 32 | 10 | CH2CH2CH2 | Ph | OEt | 59.15 | 206.41 | 3.49 | |
| 33 | 11 | 1,2,3,4-tetrahydronaphthalen-1-yl | OEt | 36.64 | 132.92 | 3.63 | ||
| 34 | 12 | propyl | OEt | 352.81 | >719.42 | >2.03 | ||
| 35 | 13 | t-butyl | OEt | 119.38 | >684.93 | >5.74 | ||
| 36 | AZTe | - | - | - | 0.0085 | 3779 | 444588 | |
bCC50 (50% cytotoxic concentration), concentration of drug that causes 50%reduction in total C8166 cell number.
cEC50 (50% effective concentration), concentration of drug that reduces syncytiaformation by 50%.
d In vitro therapeutic index (CC50 value/EC50 value). eAZT was used as a positive control.