Note that the T-shaped interaction is also present in the second aromatic pair towards the termini.
 |
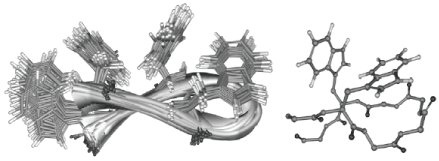
| Figure 3: (Left) Superposition of 20 best structures calculated for the
tryptophan zipper peptide using NMR (PDB code: 1LE0) [13,14]. Two pairs of
strong aromatic interactions at the non-hydrogen bonding position of the hairpin
were found to stabilize the structure and impart protein-like properties to
the 16-residue peptide. (Right) Illustration of the T-shaped aromatic interaction
in 1LE0, using the Trp residues immediately after the turn as an example. Note that the T-shaped interaction is also present in the second aromatic pair towards the termini. |