 |
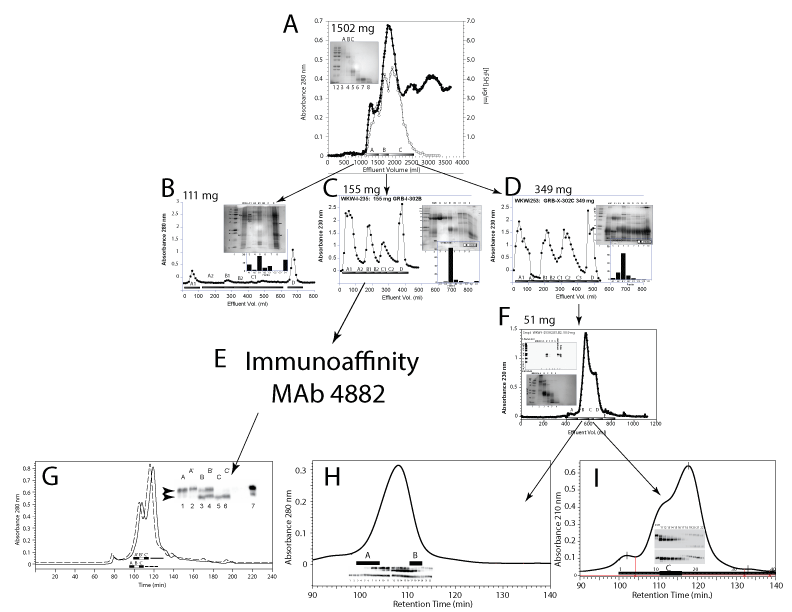
| Figure 1: Isolation of hFSH glycoform preparations, FSH24 and FSH21. A. Sephacryl G-100 chromatography of 1502 mg soluble human pituitary glycoprotein fraction on a 5 x 200 cm column equilibrated in 0.126 M ammonium bicarbonate at a flow rate of 150 mL/h. FSH was found in fractions A-C by RIA (dotted squares). B. A 111 mg sample of G-100A was chromatographed on a 5 x 15 cm phenyl-Sepharose column. The chromatogram was developed with: 1 M ammonium sulfate in 0.05 M sodium phosphate buffer, pH 7.0, followed by 0.2 M ammonium sulfate in 0.05 M sodium phosphate buffer, pH 7.0, 0.05 M sodium phosphate buffer, pH 7.0, and 40% ethanol/ water. FSH emerged in fractions A, B, and D (inset bar graph). C. A 155 mg sample of G-100B was applied to the phenyl-Sepharose column and the chromatogram developed as in Fig. 1B. FSH largely emerged in Fraction B1 (inset bar graph). D. A 349 mg sample of G100-C was applied to the phenyl-Sepharose column. FSH was recovered in fractions A2, B1 and B2 (inset bar graph). E. FSH in fraction B1 from panel C was immunopurified using anti-hFSH monoclonal antibody 4882. F. A 51 mg sample of fractions A2, B1, and B2 from panel D was was applied to a 2.5 x 200 cm Sephacryl S-100 and the chromatogram developed with with 0.126 M ammonium bicarbonate buffer at a flow rate of 45 mL/h. FSH was found in fractions B and C (upper inset). G-I. Triple-Superdex 75 gel filtration of purified hFSH. Three 1 x 30 cm Superdex 75 columns were connected in series and equilibrated with 0.2 M ammonium bicarbonate containing 20% acetonitrile. The chromatogram was developed with the same buffer at a flow rate of 0.3 mL/min. Individual fractions were collected and samples of each fraction associated with the FSH peak analyzed by FSH (panel G inset) or FSH? and FSH?-specific Western blots (panels H and I, insets). |