 |
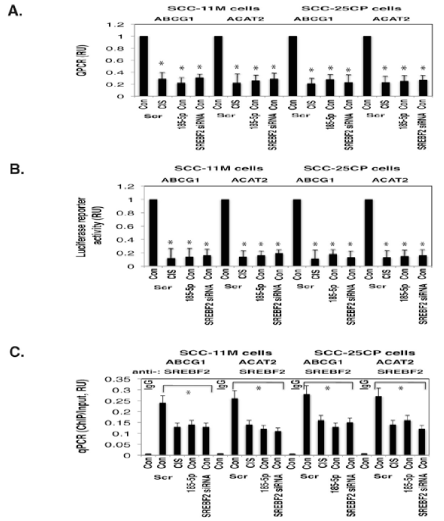
| Figure 6: Expression of ABCG1 and ACAT2 in SCC-11M cells and SCC- 25CP cells upon cisplatin exposure. SCC-11M cells and SCC-25CP cells were transfected with the scrambled (Scr) miRNA for 36h, and then exposed to control medium (Con) or 10μg/ml cisplatin (CIS) for an additional 16hours. Cells were also transfected with the miR-185-5p mimic, or SREBF2 siRNA and exposed to control media for 48hours.(A). QPCR assay for the ABCG1 and ACAT2 expression was performed from three independent experiments in triplicate (*, p<0.05). Data presented as relative values (RU) to data obtained from the control samples (SCC-11M cells transfected with the scrambled RNA and exposed to control media) designated as 1.(B). Cells were additionally transfected with 100ng of the LightSwitch_Pro reporter plasmid for the ABCG1 and ACAT2 promoters for 24hours. Renilla luciferase reporter activity assay was conducted from three independent experiments in triplicate (*, p < 0.05). Data presented as relative values (RU) to data obtained from the control samples (SCC-11M cells transfected with the scrambled RNA and exposed to control media) designated as 1.(C). ChIP-qPCR assay of the SREBF2 binding to the specific region of the ABCG1 and ACAT2 promoters. Negative controls were the normal IgG binding to the specific regions of the ABCG1 and ACAT2 promoters, as well as the SREBF2 binding to the non-specific regions. QPCR assay was performed using three independent experiments in triplicate (*, p<0.05). The amount of ChIP-enriched DNA (ChIP/Input) represented as a signal relative to the total amount of chromatin DNA (Input) using the same primers. |