 |
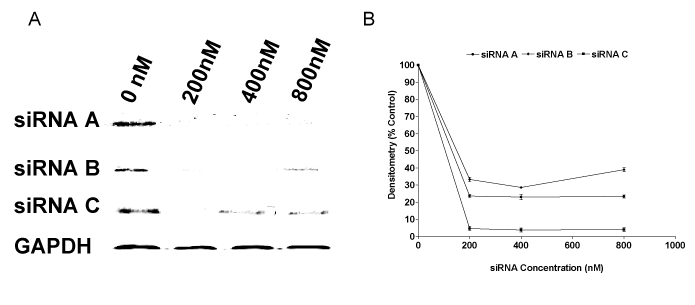
| Figure 1: siRNA sequences inhibit CCTα protein expression in A549 cells. A549 cells cultured to 80% confluence were transfected with CCTα siRNA A, B and C (0–800 nM), preincubated with FUGENE 6 transfection reagent at room temperature as indicated above. After 24 hr incubation, cell suspensions were centrifuged at 100g and cell pellets resuspended in lysis buffer (20 mM Tris, pH 7.4, 2 mM EDTA, 150 mM NaCl, 0.5% Triton X-100 and one tablet/10 ml of protease inhibitor cocktail). Samples were sonicated for 3 s, centrifuged (5 min, 4°C at 1000 rpm) and supernatants collected for total protein content analysis. Cell lysates were separated by electrophoresis on 10% SDS–polyacrylamide gels (20μg protein/lane) and then transferred to Immun-Blot PVDF membrane. Blots were blocked at 4°C overnight in 5% Carnation Instant Milk in PBS containing 0.05% Tween 20 (PBST) and then incubated overnight at 4°C with 5 μg/ml mouse anti-human CCTα affinity purified antibody. Membranes were washed three times with PBST, incubated with 1:1500 goat anti-mouse IgG-horse radish peroxidase in PBST for 3 hrs then washed three times in PBST. Immun-Blot images were obtained using a Flour-s Max Multimager. As shown in figure 1A, all the CCTα-siRNA sequences significantly inhibited CCTα protein expression with the disappearance of CCTa bands as CCTα-siRNAs were added. Figure 1B shows corresponding reduced densitometry of CCTa-siRNA treated sales as a ratio of GAPDH-siRNA. |