 |
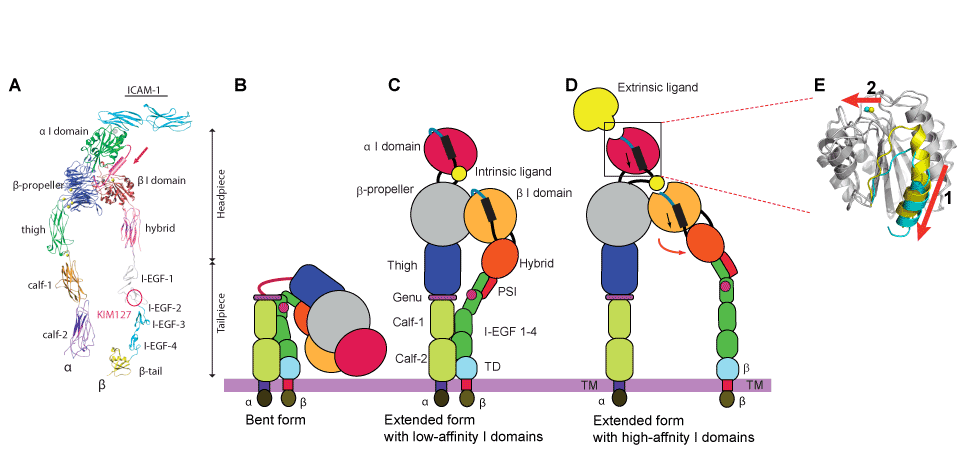
| Figure 4: Integrin structures and domains and conformational changes. (A) Integrin extracellular segment model. (B-E) Global conformational changes between the bent (B), intermediate (C), and extended (D) conformations. Blow-ups (E) showing the structures of the high- and low-affinity conformations of the alpha I domain. A piston-like downward shift of the C-terminal helix (arrow 1) is allosterically linked to the conversion of the MIDAS to the high-affinity configuration (arrow 2). Superposition of the high- (blue) and closed low- (yellow) affinity I domain is shown. Regions undergoing significant conformational changes are colored, whereas regions not undergoing significant conformational changes are in gray. |