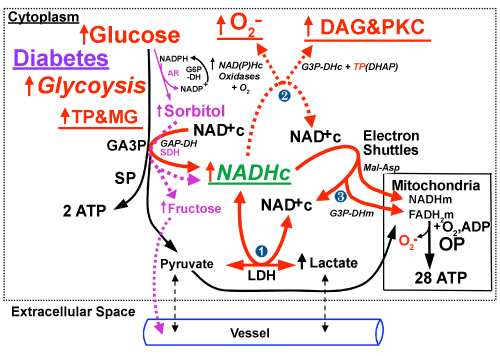
The percentage increase in sorbitol oxidation in normal rat retinas and endoneuria incubated in 30 vs. 5 mM glucose (manifested by production of fructose equimolar to NADHc) exceeds the percent increase in glycolysis (manifested by lactate production) [27,80].
Since oxidation of sorbitol does not form pyruvate, NADHc levels rise ~1.5-2-fold (manifested by increased ratios of NADH/NAD+c=lactate/pyruvate × KLDH) [44], fructose levels increase (~10-30-fold), and much more NADHc is reoxidized to NAD+c via alternative pathways, i.e. by NADHc-fueled NAD(P)H oxidases that increase superoxide production and by G3P-DHc which increases synthesis of diacylglycerol (DAG) [25-57] which activates protein kinase C (PKC) that activates NADPH-driven oxidases to further augment superoxide production. The ~1.5-2-fold increases in NADHc also contribute to product inhibition of GA3P-DH, which increases levels of GA3P and DHAP, i.e. triose phosphates- TPs, [25] that are in equilibrium. Non-enzymatic degradation of DHAP and GA3P forms methylglyoxal (MG), a precursor of advanced glycation endproducts (AGEs) that impair the activity of numerous enzymes including GA3PDH [25,61,62].