 |
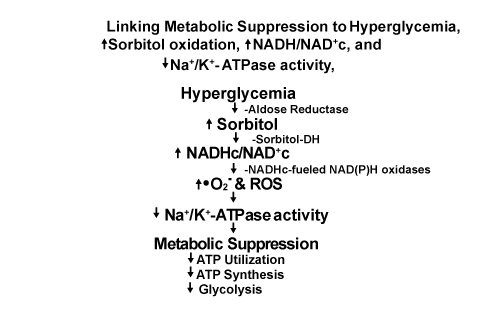
| Figure 3: Sorbitol oxidation, Oxidative stress, impaired Na+/K+-ATPase activity, elevated plasma NEFA levels: links to Insulin Resistance and Metabolic suppression manifested by decreased glucose utilization, impaired ATP synthesis, and decreased glycolysis. Increased levels of reduced NADHc 1) fuel production of superoxide (•O2-) and related Reactive Oxygen Species (ROS) by NADHc-fueled NAD(P)H oxidases coupled to reoxidation of NADHc to NAD+c and 2) increase triose phosphates, including DHAP which is reduced to G3P by G3P-DH coupled to reoxidation of NADHc to NAD+c, the first step in one pathway for de novo synthesis of diacylglycerol (DAG) that activates PKC which, in turn, activates some isoforms of NAD(P)H-driven oxidases that further augment superoxide production (Figure 1). Increased superoxide and ROS impair Na+/K+-ATPase activity which preferentially limits utilization of ATP by numerous enzymes and metabolic pathways not crucial for sustaining viability while selectively maintaining utilization of ATP by enzymes and metabolic pathways vital for sustaining viability, e.g. Na+/K+-ATPase activity which maintains high intracellular ratios of K+/Na+ versus high extracellular ratios of Na+/K+. These changes result in decreased overall rates of ATP utilization, ATP synthesis, and glycolysis, i.e. Metabolic Suppression, consistent with the hypothesis of Hochachka [70-72] and beneficial effects of Hypoxic/Ischemic Preconditioning (HIP). Utilization of glucose by insulin-sensitive tissues in type 2 diabetics, e.g. skeletal muscle, liver, fat cells, etc. requires binding of insulin to cell surface receptors followed by tyrosine phosphorylation of IRS-1 which mediates insulin effects on glucose metabolism [100]. Phosphorylation of IRS-1 is significantly impaired in obese nondiabetic subjects and in type 2 diabetics resulting in insulin resistance [100]. Intravascular infusion of NEFA in nondiabetics to reproduce plasma NEFA levels in type 2 diabetes also causes muscle and liver insulin resistance and inhibits insulin secretion: “the 3 basic core metabolic defects in type 2 diabetes” [100]. DeFronzo [100] attributes insulin resistance in muscle and liver in type 2 diabetics primarily to sequelae of impaired phosphorylation of tyrosine residues on the beta chain of IRS-1 causing downstream impairment of glucose metabolism in numerous tissues. We suggest this impairment of glucose metabolism is consistent with Metabolic Suppression, i.e. ↓ATP utilization, ↓ATP synthesis, and ↓glycolysis. This scenario is consistent with downregulation of enzymatic reactions that utilize ATP for phosphorylation of IRS-1 and other reactions not crucial for maintaining cell viability ‘in the short term’. This scenario also is consistent with Metabolic Suppression evoked by hypoxia (and, we suggest, ‘Hyperglycemic pseudohypoxia’ [46]), and beneficial effects of hypoxic/ischemic preconditioning (HIP). |