 |
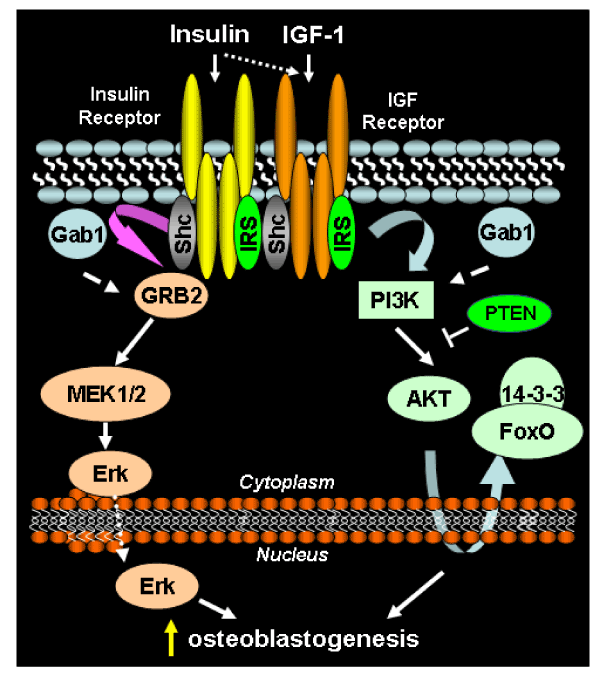
| Figure 1: Pro-osteoblastogenic signal transduction by insulin and IGF1 receptors. Receptors at the plasma membrane bind ligands within extracellular domains, triggering receptor autophosphorylation on tyrosine residues within the cytoplasmic domain. Phosphorylated receptors recruit scaffolding proteins [src homology 2 domain-containing transforming protein C (Shc), IRS], which are subsequently phosphorylated by the receptor kinase domain. Recruitment of growth factor receptor bound protein 2 (GRB2) to the receptor-Shc complex initiates phosphorylation and activation of the MEK/ERK pathway. MEK/ERK signaling culminates with phosphorylation of osteoblastogenic transcription factors in the nucleus. IRS proteins recruit phosphatidylinositol 3-kinase to the receptor complex, resulting in kinase activation, which generates phosphatidylinositol (3,4,5) triphosphate (PIP3). PIP3 recruits AKT to the plasma membrane, resulting in AKT activation. Activated AKT phosphorylates numerous proteins, among them FoxO1, resulting in retention of FoxO1 in the cytosol, where it is unable to perform its function as a transcription factor. |