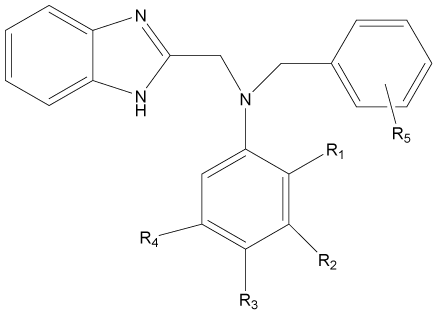
| S. No | R1 | R2 | R3 | R4 | R5 | MICa | MICb | MICc | MICd |
| 1 | F | H | H | H | H | 4.798 | 4.795 | 4.494 | 4.494 |
| 2 | H | CF3 | H | H | H | 4.795 | 4.494 | 4.494 | 4.494 |
| 3* | H | H | F | H | H | 4.494 | 4.193 | 4.795 | 4.795 |
| 4 | H | H | Cl | H | H | 4.193 | 4.494 | 4.494 | 4.795 |
| 5 | H | H | I | H | H | 4.193 | 3.892 | 4.795 | 4.795 |
| 6 | H | H | CH3 | H | H | 4.494 | 3.290 | 4.795 | 4.494 |
| 7 | F | H | F | H | H | 4.795 | 4.795 | 4.795 | 4.795 |
| 8* | Cl | H | Cl | H | H | 4.193 | 4.795 | 4.494 | 4.494 |
| 9 | H | CF3 | H | CF3 | H | 5.698 | 5.096 | 5.698 | 5.698 |
| 10 |  |
H | H | H | H | 3.892 | 3.892 | 3.892 | 3.892 |
| 11 | 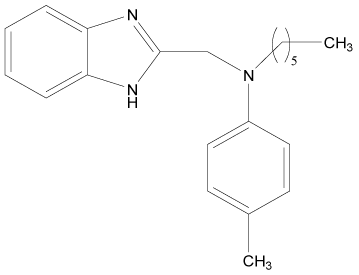 |
H | H | H | H | 3.892 | 4.795 | 3.591 | 4.795 |
| 12* |  |
H | H | H | H | 4.494 | 3.892 | 3.892 | 4.795 |
| 13 |  |
H | H | H | H | 3.892 | 3.892 | 3.892 | 4.795 |
| 14 |  |
H | H | H | H | 4.193 | 4.795 | 3.892 | 4.795 |
| 15 |  |
H | H | H | H | 3.892 | 3.591 | 3.591 | 4.494 |
| 16 | F | H | H | H | 3, 4-2 Cl | 4.193 | 3.892 | 4.494 | 4.494 |
| 17* | H | CF3 | H | H | 2, 4-2 Cl | 4.494 | 4.494 | 4.795 | 4.795 |
| 18 | H | H | F | H | 2, 4-2 Cl | 4.494 | 4.795 | 3.892 | 4.494 |
| 19 | H | H | CH3 | H | 2-Cl | 4.193 | 3.892 | 4.494 | 4.494 |
| 20 | H | H | CH3 | H | 3-Cl | 4.494 | 3.892 | 4.494 | 4.494 |
| 21* | H | H | CH3 | H | 4-Cl | 4.494 | 3.892 | 4.494 | 4.795 |
| 22 | H | H | CH3 | H | 4-NO2 | 4.494 | 3.892 | 4.795 | 3.892 |
| 23 | H | H | CH3 | H | 2, 4-2F | 4.795 | 4.795 | 3.892 | 4.494 |
| 24 | H | H | CH3 | H | 2, 4-2 Cl | 4.193 | 3.892 | 4.494 | 4.795 |
| 25 | H | H | CH3 | H | 3, 4-2 Cl | 4.494 | 4.795 | 4.795 | 3.892 |
| 26 | F | H | F | H | 2, 4-2 Cl | 4.193 | 4.795 | 4.494 | 3.892 |
| 27 | Cl | H | Cl | H | 2, 4-2F | 4.193 | 3.892 | 4.795 | 3.892 |
| 28* | H | CF3 | H | CF3 | 2, 4-2F | 5.096 | 5.096 | 4.795 | 4.795 |
| 29 | H | CF3 | H | CF3 | 2, 4-2 Cl | 4.193 | 3.892 | 4.795 | 4.795 |
| 30 |  |
H | H | H | H | 4.193 | 4.795 | 3.892 | 4.795 |
| 31 |  |
H | H | H | H | 5.096 | 5.096 | 4.494 | 5.096 |
| 32* |  |
H | H | H | H | 4.494 | 3.892 | 3.892 | 4.795 |
| 33 |  |
H | H | H | H | 3.892 | 3.892 | 3.892 | 4.795 |
| 34 |  |
H | H | H | H | 4.193 | 3.892 | 3.892 | 4.494 |
aStaphylococcus aureus; bMicrococcus luteus; cEscherichia coli; dCandida albicans