 |
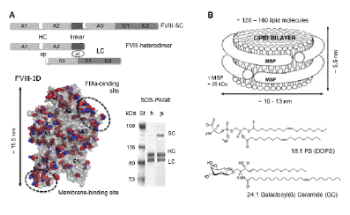
| Figure 1: A. Domain organization of recombinant human (h) and porcine (p) FVIII lacking the B domain. The heavy (HC) and light (LC) chains are linked with a peptide of 24 amino acid (aa) residues for the porcine and 21 aa residues for the human FVIII single chain (SC) forms. The HC and LC are linked electrostatically (el) in the FVIII heterodimer, which is the predominant form of FVIII in vitro , as shown on the SDS-polyacrylamide gel (PAGE). In white are shown the activation peptides (ap). The hFVIII crystal structure (FVIII-3D, 3CDZ.PDB) is shown as a grey surface. The side chains of the aa residues of the hFVIII, which differ from the pFVIII are shown as red spheres and the side chains of the aa residues of the pFVIII that differ from the hFVIII are shown as blue spheres. The FVIII domains are indicated, as well as the main membrane- and FIXa-binding sites. B. Schematic of a lipid nanodiscs (ND) circled by two membrane scaffolding proteins (MSP). The chemical structure of the lipids employed for the ND in this study: phosphatidylserine (PS) and galactosylceramide (GC) are also shown. |