 |
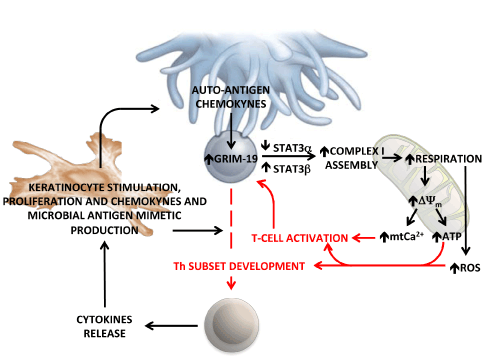
| Figure 7: Schematic representation of the proposed role of mitochondrial OxPhos in the development of psoriasis. Naïve T-lymphocyte is shown to form a synaptic interaction with an (auto)-antigen presenting cell. Following the interaction and chemokynes-mediated activation, the expression of GRIM-19 is up-regulated leading to enhanced assembly of the mitochondrial complex I. This process possibly requires mitochondria-localized STAT3. The in-this-study-observed shift in the relative expression of the α and β STAT3 splisoforms is also shown. The ensued increase of the respiratory chain activity and of the linked membrane potential (ΔΨm) provides the driving force for the synthesis of ATP and for the uptake of Ca2+ within the mitochondria. This latter process promotes T-cell activation. An additional effect of the enhanced respiratory activity might be an over-production of reactive oxygen species (ROS), which by redox signalling fosters the differentiation of specific T-helper subsets. These in turn produce cytokines that activate keratinocytes. The keratinocytes (and psoriatic lesions) proliferate and produce a variety of cytokines, chemokines and antimicrobial peptides that feed back into the inflammatory cascade, forming an autoinflammatory loop. See Discussion for further details and references. |