 |
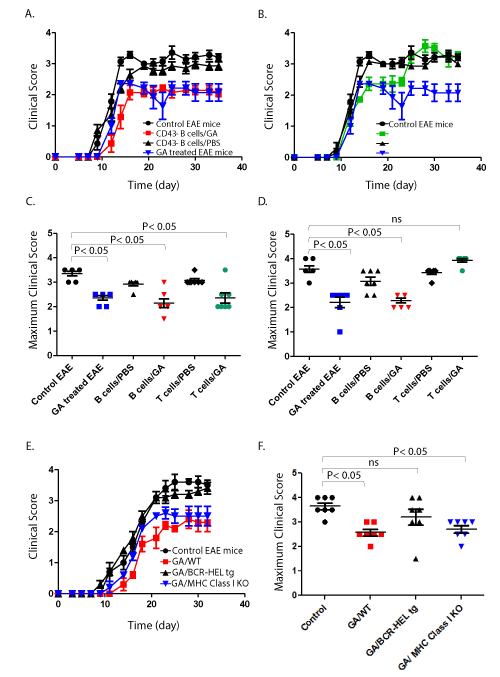
| Figure 4: B lymphocytes maintain the efficacy observed with GA treatment in EAE and BCR recognition of GA is required. Wild-type C57Bl/6 mice were treated with 200 μg GA or PBS s.c. for 14 days. Spleen and draining lymph nodes were removed and CD43- B cells or CD4+ T cells were purified by negative selection and transferred i.p. to wild-type C57Bl/6 mice on the day of EAE induction. The control EAE mice were injected with PBS daily, while the GA treated mice received 200 μg s.c. daily. Animals were given a score based on motor function as described in the materials and methods every other day. (A and B) The transfer of both CD43- B lymphocytes and CD4+ T lymphocytes from GA treated animals is initially equally efficacious in the EAE model. However, animals that received CD4+ T lymphocytes lose this benefit 21 days post disease induction. Shown is the clinical score based on motor function for the indicated mice. (B) The maximum clinical score achieved for the indicated group of mice prior to day 21. (C) The maximum clinical score achieved for the indicated group of mice post day 21. (E and F) Wild-type C57Bl/6, BCR-HEL tg, or MHC Class I deficient mice were treated with 200 μg GA or PBS s.c. for 14 days. CD43- splenic B cells were purified by negative selection and transferred i.p. to wild-type C57Bl/6 mice on the day of EAE induction. (E) The clinical score over time as measured by impairment in motor function (F) The maximum clinical score achieved throughout the duration of the study. Data is representative of two experiments with similar results performed with 7 mice per group. Statistical analysis was performed using a one-way ANOVA followed by a Bonferroni post-test. |