 |
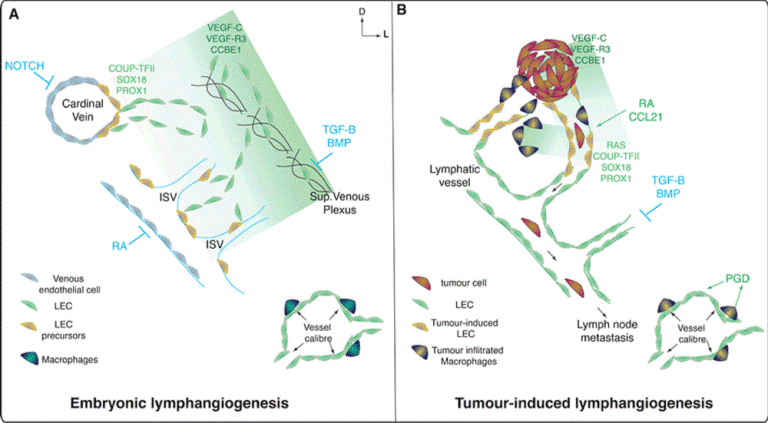
| Figure 1: “Ordered chaos”: Key developmental pathways activated during embryonic development face re-activation under tumor conditions to drive aberrant lymphangiogenesis and metastatic spread. (A) Schematic representation of embryonic lymphangiogenesis and the major genetic pathways that instruct lymphatic endothelial cell fate (SOX18, COUP-TFII, PROX1), maintain venous identity (Notch, RA) and govern morphogenesis of the lymphatic vascular plexus (VEGF-C, VEGF- R3, CCBE1, TGF-B, BMP9 and BMP2) from 9 dpc-14.5 dpc in mice. During development venous endothelial cells provide LEC precursor cells and macrophages control lymphatic vessel calibre. (B) Under tumor-induced conditions, embryonic pathways are re-activated or dysregulated to cause expansion of the pre-existing lymphatic vasculature in and around tumor tissues. Neo-lymphatics form an ‘on- ramp’ for tumor cells and fluid draining to the lymph node basin. Reactivation of the developmental program is influenced by the inflammatory response, components like tumor-infiltrated macrophages produce VEGF-C and change their molecular profile to acquire a LEC-like molecular signature. These factors contribute to produce a pseudo-functional lymphatic vasculature. |