 |
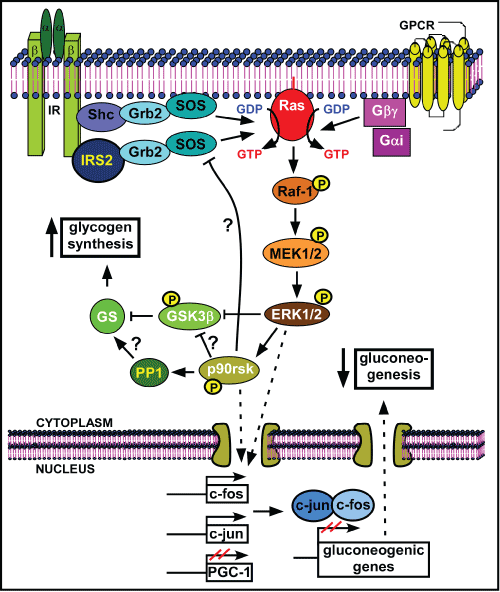
| Figure 1: NaW signaling pathway in liver. NaW does not directly activate IR, or other tyrosine kinase receptors, such as EGF or IGF receptors in hepatocytes. Rather, it activates Gαi2 and Gβγ subunits of GPCR, to stimulate the activity of the small GTPase Ras, initiating the signaling transduction through sequential phosphorylation of Raf-1, MEK1/2 and ERK1/2, which in turn phosphorylates GSK3β and p90rsk. Phosphorylated GSK3β is inactive and alleviates the inhibitory phosphorylation over GS, favoring activation of glycogen synthesis. Although it has not been demonstrated for NaW action, p90rsk is known to be involved in GSK3β inactivation, PP1 activation and negative feedback upstream in the pathway through inactivation of SOS. PP1 is the main phosphatase activity participating in GS dephosphorylation and stimulation of glycogen synthesis, but the actual participation of NaW in PP1 activation remains to be established. In parallel, NaW-madiated ERK1/2 activation stimulates c-fos and c-jun, and inhibits PGC-1 transcriptional expression through yet unknown mechanisms. Since PGC-1 is a positive modulator, whereas c-fos and c-jun are negative regulators of the transactivation of gluconeogenic genes, NaW action finally induces down-regulation of gluconeogenic enzymes and, hence, the gluconeogenic pathway. In IRS2 KO mice, NaW was unable to exert consistent effects on glycogen synthesis and gluconeogenesis, highlighting the importance of IRS2 in NaW signaling pathway in hepatocytes. White letter: proteins are modified after NaW treatment. Black letter: proteins involved in the normal pathway, which are expected to participate in NaW signaling, although it has not been determined; Yellow letter: evidence supports these proteins as potentially involved in NaW signalling; ?: regulatory mechanisms that have not been confirmed for NaW signalling; P: phosphorylation. |