 |
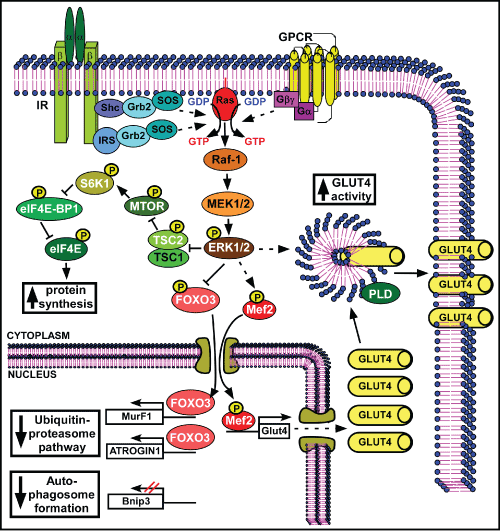
| Figure 2: NaW signaling pathway in muscle. There is no available information regarding NaW signaling upstream of ERK1/2 in muscle, except that again IR is not activated. The same scheme proposed for liver upstream of ERK1/2 (Scheme 1) was used as a reference. NaW induced-ERK1/2 activation led to phosphorylation and inhibition of TSC2, relieving inhibition on MTOR. Activated MTOR phosphorylates and activates S6K1, which inhibits eIF4EBP1, alleviating eIF4E inhibition, and promoting protein translation. Moreover, ERK1/2 phosphorylates FOXO3 and Mef2 transcription factors. Phosphorylated FOXO3 is inactive and cannot translocate into the nucleus to transactivate MurF1 and ATROGIN1 genes, down-regulating the expression of these important ubiquitin ligases, and reducing the activity of the ubiquitin-proteasome pathway of protein degradation. Besides, NaW induces a decline in auto-phagosome formation through the down-regulation of Bnip3. On the other hand, phosphorylated Mef2 is active and can enter the nucleus to promote Glut4 gene expression, which together with above-mentioned eIF4E stimulation led to enhanced protein synthesis of GLUT4 transporter. Finally, NaW-induced ERK1/2 activation stimulates fusion of GLUT4-containing vesicles with the plasma membrane, maybe through direct activation of PLD, enhancing GLUT4 activity and glucose uptake. White letter: proteins are modified after NaW treatment; Black letter: proteins involved in the normal pathway, which are expected to be involved in NaW signaling, although it has not been determined; Yellow letter: evidence supports these proteins as potentially involved in NaW signalling; ?: regulatory mechanisms that have not been confirmed for NaW signalling, P: phosphorylation. |