 |
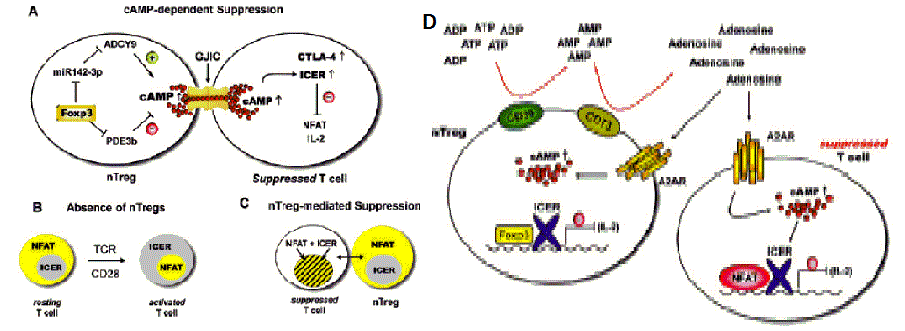
| Figure 7: nTreg cells direct ICER into the nucleus of activated CD4+ Tcons via cAMP [55]. (A) ‘Supraphysiologically’ high intracellular cAMP levels are generated in nTreg cells, at least in part, by Foxp3-mediated down-regulation of the Pde3b gene. Furthermore, Foxp3 also downregulates miR-142-3p targeting adenylyl cyclase (ADCY9) mRNA resulting in up-regulated cAMP production [76,77]. cAMP is then transferred from nTreg cells to the activated conventional CD4+ T cells via gap junction intercellular communications (GJICs) [49]. There cAMP has at least two effects: first it induces ICER expression and second it enables the nuclear localization of ICER leading to transcriptional attenuation of IL-2 synthesis by suppression of NFATc1/α gene expression and/or formation of inhibitory NFAT/ICER protein complexes responsible for attenuated 23 expression of IL-2 and numerous other NFAT-driven cytokines and chemokines [55]. In addition, cAMP may up-regulate surface expression of CTLA-4 in suppressed conventional CD4+ T cells [74], thus conferring a B7 inhibitory signal to target cell populations [52,53]. (B) In the absence of nTreg cells, for example, after ablation of nTreg in DEREG mice, TCR triggering and CD28- costimulation via CD28 superagonist (CD28SA) mAb results in cytosolic localization of ICER, which disables its function as a transcriptional repressor leading to unopposed NFAT-driven transcription [55]. When ICER is ousted to the cytosol, NFAT is translocated into nucleus and drives vigorous IL-2 expression in Tcons upon CD28 co-stimulation (activated T cell) [55]. (C) Gap junction intercellular communications (GJICs) transferring cAMP from nTreg cells to Tcons lead to the maintenance of ICER in the nucleus of both cell populations during nTregcell- mediated suppression [49]. In the presence of a CD28 signal (either CD3/CD28 in vitro or CD28SA in vivo ), ICER and NFAT co-localize in the nucleus of activated Tcons, nuclear co-localization of ICER and NFAT leads to the inhibition of NFATc1/α gene induction and/or formation of inhibitory NFAT/ICER protein complexes, thus inhibiting NFAT-driven transcription of IL-2 and numerous other cytokines and chemokines [44]. (D) Hypoxia-adenosinergic signaling: An additional model for the cAMP-enabled and nTreg-cell-mediated suppressive function of ICER, CD39, and CD73 ectoenzymes on nTreg cells. These cells generate extracellular immunosuppressive adenosine from ATP, which adds to the suppressive effects of inflamed-tissue hypoxic adenosinergic signaling on conventional CD4+ T cells [78,79] acting via the A2A receptor (A2AR) expressed on CD4+ T cells (both in nTreg cells and Tcons). A2AR signaling enhances the levels of intracellular cAMP and, presumably, in synergy with the model described in Figure 6 enforces nuclear localization of ICER leading to transcriptional attenuation of IL-2 production in suppressed Tcons [54,55]. |