 |
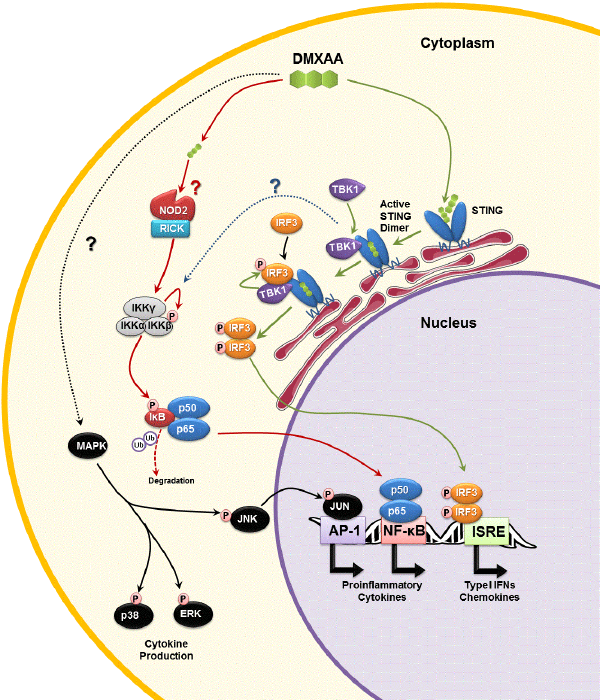
| Figure 3: Molecular Pathways of DMXAA’s cytokine inducingactivity in mice. DMXAA binds and activates STING in thecytoplasm of mouse macrophages (green arrows) [40,55]. ActivatedSTING dimers recruit TBK1, which in turn phosphorylate (P)IRF3, and possibly inhibitor of NF-κB (IκB) kinase complex (IKK)(dotted blue line) [26]. Phosphorylated IRF3 dimerizes andtranslocates to the nucleus where it is recognizes interferonstimulatedresponse elements (ISRE), triggering expression of type Iinterferons and chemokines. DMXAA can also activate NF-κBthrough either NOD1 or NOD2, in a RICK-dependent manner (redarrows) [57]. RICK activates the IKK complex which triggersubiquitin (Ub)-mediated degradation of IκB, thereby releasing NF-κB (p50/p65). Activated NF-κB translocates to the nucleus toinduce expression of NF-κB-responsive genes (i.e. proinflammatorycytokines and chemokines). DMXAA also triggers activation ofmultiple MAPK kinase signaling pathways, through unknownRICK-independent mechanism (dotted black line) [58]. Activationof p38 and ERK pathways are important for the secretion ofproinflammatory cytokines (e.g. TNF-α and IL-6), but not geneexpression [58]. Jun-kinase (JNK) is also phosphorylated followingexposure to DMXAA, without impacting on TNF-α or IL-6production. JNK’s role is to phosphorylate JUN, a component ofthe activator protein-1 (AP-1) complex. NF-κB and AP-1 are bothessential for maximal production of IL-8 [66], which although notobserved in mice, may explain the induction of IL-8 by humanactiveXAA analogues in human leukocytes [63]. ‘?’ denote unclearmechanisms. |