 |
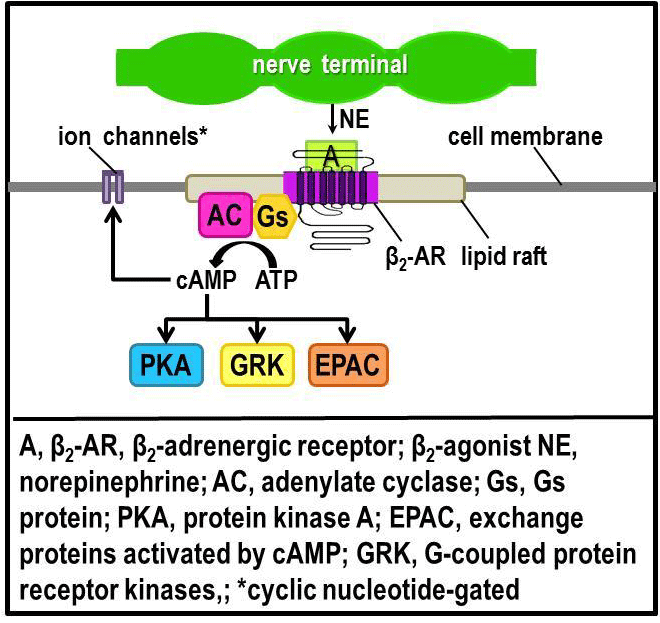
| Figure 2: β2-AR-mediated signal transduction in immune organs. Norepinephrine (NE) is released from sympathetic nerves. NE released from sympathetic nerves binds to β2-ARs expressed in innate and adaptive immune cells to regulate immune cell functions. Binding of NE to β2-ARs activates the G protein, Gs. Gs protein regulates the activity of adenylate cyclase (AC). Adenylate cyclase catalyzes dephosphorylation of ATP to cyclic AMP (cAMP). Cyclic AMP activates protein kinase A (PKA), G protein-coupled receptor kinases (GRK), and exchange protein activated by adenylyl cyclase (EPAC). PKA is a kinase that regulates the activity of several proteins by their phosphorylation. PKA phosphorylation of target proteins is responsible for many of the β2-ARinduced cellular responses. Activation of GRK ultimately determines the later functions and fate of β2-ARs, including the induction of alternative intracellular signaling, and receptor desensitization, internalization, recycling and/or trafficking to the lysosomes for degradation. Activation of exchange protein directly activated by cAMP (EPAC) leads to activation of the B-Raf/mitogenactivated protein kinase signaling pathway. Downstream effects of PKA, GRK and EPAC regulate diverse cellular processes, including gene transcription by transcription factors. Additionally, cAMP can bind to their binding sites on ion channels that express cAMP binding sites. Binding of cAMP to these cyclic nucleotides-gated ion channels then alter movement of ions across the channel to exert change in cell functions. |