 |
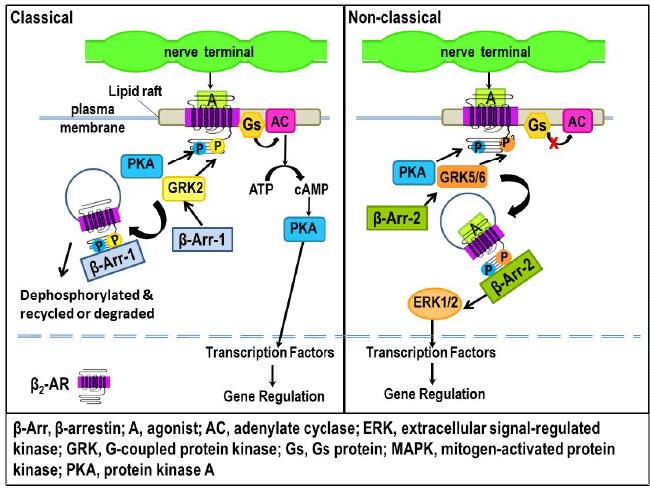
| Figure 17: A-B. Classical and non-classical signaling of β2-ARs. A) Agonist binding to β2-ARs causes the dissociation of Gs subunits (Gsα and βγ). The Gsα subunit activates AC, which catalyzes the conversion of ATP to cAMP and the subsequent cAMP-induced activation of PKA. PKA activates transcription factors that subsequently regulate gene transcription to alter immune cell function. Receptor function is terminated by GRK2 phosphorylation of the receptor, which causes an uncoupling of Gs to AC. GRK2 recruits β-arrestin-1 to the receptor. Receptors phosphorylated by PKA and GRK2 are internalized via functions of β-arrestin-1. The internalized receptor is then either dephosphorylated and recycled to the membrane or transported to lysosomes for degradation. B) Under conditions of high agonist concentration, β2-ARs can be phosphorylated by GRK5/6 rather than GRK2. Phosphorylation of β2-AR by GRKs 5 or 6 desensitizes receptor signaling via cAMP-PKA by uncoupling the receptor from Gs. Phosphorylation of the receptor by GRK5/6 also induces β2-AR internalization by recruiting β-arrestin-2 to the receptor. Once bound to the receptor, β-arrestin-2 acts as a scaffold for the sustained activation of the MAPK, ERK 1/2. Beta-arrestin-2 activation of ERK1/2 MAPK, in turn, increases the translocation of transcription factors into the nucleus to influence gene transcription and thus, alter cell functions. |