 |
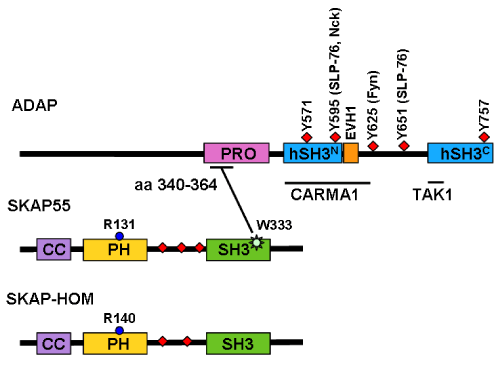
| Figure 1: Structure of ADAP, SKAP55, and SKAP-HOM. Amino acid (aa) numbering are given for human ADAP isoform 2 (NM1999335.3), SKAP55 (Y11215.1) and SKAP-HOM (AJ004886.1). ADAP has a N-terminal prolinerich domain (PRO), multiple tyrosine-based signaling motifs, two helical SH3 domains (hSH3), an Ena-VASP homology 1 (EVH1)-binding site, and binding sites for CARMA1 (caspase recruitment domain (CARD) membrane-associated guanylate kinase (MAGUK) protein 1) as well as for TAK1 (transforming growth factor-β (TGFβ)-activated protein kinase). The amino acids stretch 340-364 within the proline-rich domain of ADAP interacts with the tryptophan (W) at position 333 within the SH3 domain of SKAP55. SKAP55 and SKAP-HOM, both contain a central PH domain and C-terminal SH3 domain. In contrast to the three tyrosine-based signaling motifs SKAP55 (indicated as red squares), SKAP-HOM possesses only two of these motifs. Both SKAP-HOM and SKAP55 contain a coiled-coil region (CC) important for dimerization and an arginine (R) at position 140/131 within the PH domain that binds probably to PIP3. |