 |
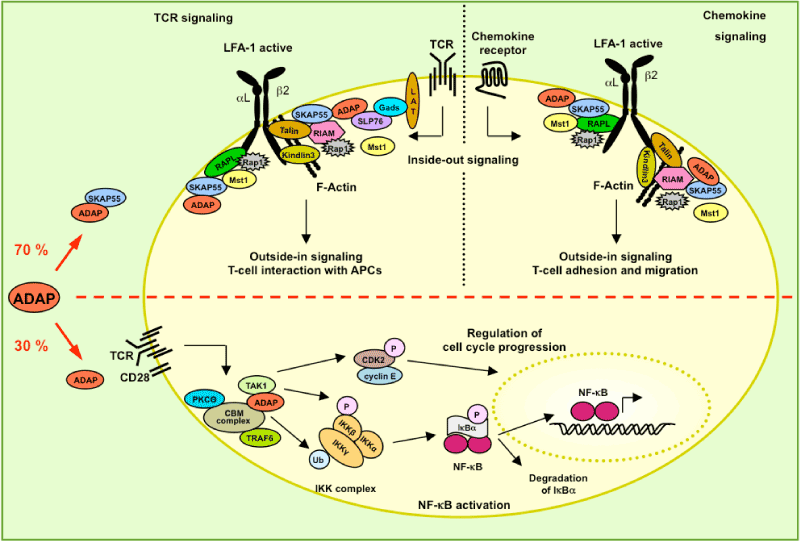
| Figure 2: Schematic model of how the two distinct pools of ADAP coordinate NF-κB activation or integrin activation. The pool of ADAP associated with SKAP55 (app. 70%) regulates integrin function. In response to chemokine receptor stimulation, the ADAP/SKAP55/RIAM/Mst1/Kindlin-3/Rap1 and the ADAP/SKAP55/RAPL/ Mst1/Rap1 appear to independently interact with the α-chain (CD11a) probably via RAPL and the β-chain (CD18) of LFA-1 via Kindlin-3 to facilitate LFA-1 inside-out/ outside-in signaling required for T-cell migration. The interaction between the Ferm-domain containing protein Talin with the ADAP/SKAP55/RIAM/Mst1/Kindlin-3/Rap1 may provide a further link to F-Actin. Similar to chemokine stimulation, upon TCR triggering, the two distinct ADAP/SKAP55/RIAM/Mst1/Kindlin-3/Rap1 and the ADAP/ SKAP55/RAPL/Mst1/Rap1 are recruited either via the inducible association between SLP-76 and ADAP or via the PIP3-binding property of the PH domain of SKAP55 to the plasma membrane in close proximity to the integrin LFA-1 to promote T-cell interaction with APCs. The pool of ADAP not associated with SKAP55 (app. 30%) is most likely involved in NF-κB activation and cell cycle progression upon TCR/CD28 stimulation. In this scenario ADAP is required for the assembly of the PKCθ/CBM/ TRAF6/ADAP/TAK1 signalosome. The presence of ADAP within this signalosome controls IKK complex activation by polyubiquitination of IKKγ by TRAF6 and IKKβ phosphorylation via TAK1. The activated IKK phosphorylates IκBα thereby facilitating the degradation of this inhibitory subunit of the NF-κB transcription factor. NF-κB now translocatesto the nucleus controls NF-κB-dependent gene expression. Besides the role of ADAP for NF-κB activation, the interaction of ADAP with CARMA1 and TAK1 regulates cyclin E and Cdk2 expression. Both the induction of cyclin E and Cdk2 are critically involved at the late G1 restriction point of the cell cycle. |