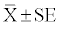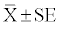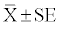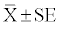| |
Study Day 7 |
| |
Animal# |
uAlb/uCr(ng/mg) |
uLpn2/uCr(ng/mg) |
uKIM-1/uCr(ng/mg) |
uOpn/uCr(ng/mg) |
| Vehicle(0.9% Saline) |
1 |
12743.8 |
170.6 |
0.9 |
3.9 |
| 2 |
10130.9 |
236.2 |
1.3 |
9.4 |
| 3 |
15509.5 |
318.4 |
0.8 |
5.3 |
| 4 |
9748.2 |
181.7 |
1.1 |
5.0 |
| 5 |
8619.3 |
193.2 |
1.0 |
7.2 |
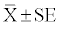 |
11350.0 ± 1240 |
220.0 ± 27.0 |
1.0 ± 0.1 |
6.2 ± 1.0 |
| Doxorubicin(5 mg/kg/dose) |
21 |
>105857.7§ |
370.3 |
0.5 |
5.0 |
| 22 |
>108817.2§ |
280.8 |
0.5 |
5.1 |
| 23 |
38808.0 |
246.6 |
0.5 |
5.7 |
| 24 |
>107431.0§ |
231.7 |
0.6 |
4.8 |
| 25 |
>126500.0§ |
269.5 |
0.4 |
7.0 |
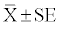 |
>97483.0 ± 15137§*** |
280.0 ± 24.2 |
0.5 ± 0.0*** |
5.5 ± 0.4 |
| Fold Δ |
>8.6§ |
1.3 |
0.5 |
0.9 |
| |
Study Day 14 |
| Vehicle(0.9% Saline) |
1 |
8078.3 |
175.8 |
0.6 |
2.7 |
| 2 |
8993.5 |
325.1 |
1.0 |
7.9 |
| 3 |
13690.8 |
216.2 |
0.7 |
2.6 |
| 4 |
11170.9 |
142.8 |
1.0 |
2.0 |
| 5 |
11382.1 |
148.9 |
0.8 |
3.8 |
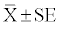 |
10663.0 ± 985.2 |
201.8 ± 33.4 |
0.8 ± 0.1 |
3.8 ± 1.1 |
| Doxorubicin(5 mg/kg/dose) |
21 |
>34776.6§ |
775.9 |
3.3 |
4.7 |
| 22 |
>98252.4§ |
678.1 |
2.5 |
4.2 |
| 23 |
>49607.8§ |
378.6 |
0.5 |
3.2 |
| 24 |
>47157.5§ |
936.3 |
3.1 |
3.9 |
| 25 |
>80063.3§ |
1334.1 |
11.89 |
5.2 |
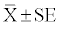 |
>353567.0 ± 136273.0§* |
820.6 ± 157.3** |
4.2 ± 1.9 |
4.2 ± 0.4 |
| Fold Δ |
>33.1§ |
4.1 |
5.3 |
1.1 |
| Urinary biomarkers (urinary albumin, uAlb; urinary lipocalin-2, uLpn2; urinary kidney injury molecule 1, KIM-1; urinary osteopontin, uOpn) were normalized to concurrent urinary creatinine (uCr) concentrations. §Doxorubicin-induced increased uAlb concentrations were above the upper limit of assay detection. Values significantly different from Vehicle (0.9% Saline) control are indicated as ***p<0.001, **p<0.01 or *p<0.05. |
|