 |
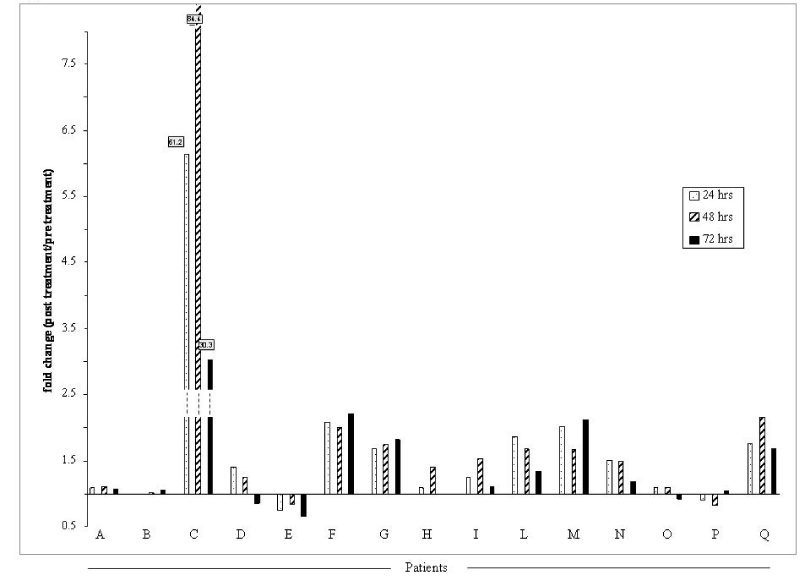
| Figure 6: Plasma GDF15 levels in patients before and after treatment with Danusertib. Plasma samples from individual patients enrolled in the phase I clinical trial where Danusertib was administered as a 24-hour infusion in a 14-day cycle, were collected predose and after 24, 48 and 72 hrs of the starting treatment. Data reported for some patients (n= 15) indicate the fold changes of the post-treatment versus pre-treatment samples for each analysed patient. |