 |
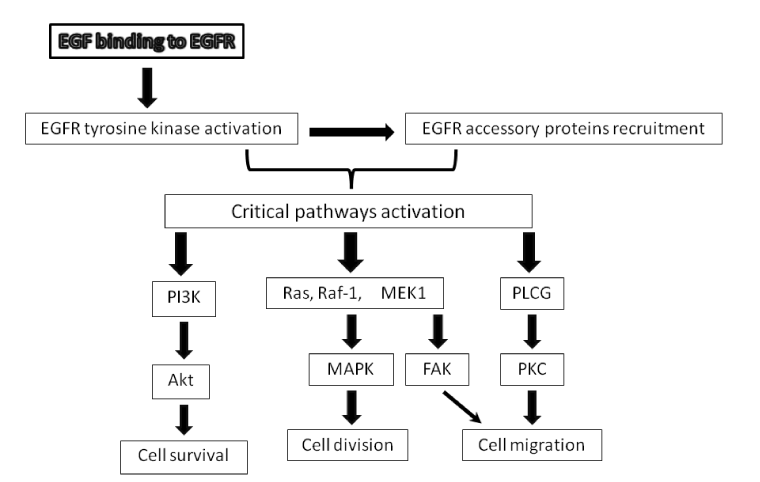
| Figure 1: Main EGFR signaling pathways in wound healing and cancer. EGFR occupation by EGF or other cognate agonistic ligand triggers a conformational change within the receptorís topography leading to carboxy-terminal tyrosines phosphorylation and accessory proteins recruitment. Three major signaling pathways have been described upon EGFR occupation. PI3K, phosphatidil inositol 3-kinase, involved in cyto-protection and cell tolerance to hypoxia. PI3K phosphorylates downstream substrates as Akt or PKB on serine 473. Consequently Akt inhibits apoptosis via BAD and BAX inactivation. This pathway assists in cell survival and appears to be involved in wound bed and tumor cells survival when angiogenesis is not accomplished, thus contributing to tumor metastasis. Cell proliferation involves the RAS-RAF-MAPK pathway, where phosphorylated EGFR recruits accessory proteins which activate the oncogene derived proteins RAS, subsequently RAF, and the Mitogen-Activated Protein Kinase (MAPK) pathway leading to cell cycle inhibitors blockade, cyclins synthesis and cell proliferation. This pathway may participate in wound bed re-population as in tumor invasion and metastasis. Phospholipase C-gamma (PLCG) activation via phosphorylation, renders the hydrolysis of phosphatidylinositol 4,5 biphosphate (PIP2) into inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), resulting in activation of protein kinase C (PKC). This pathway is involved in cell migration cooperating with focal adhesion kinase complex (FAK). Besides PKC activation is also responsible for controlling receptor downregulation and protein kinase activity by phosphorylating the yuxtamembrane residue of threonine 654. This results in a temporary inhibition of the tyrosine kinase activity. |