 |
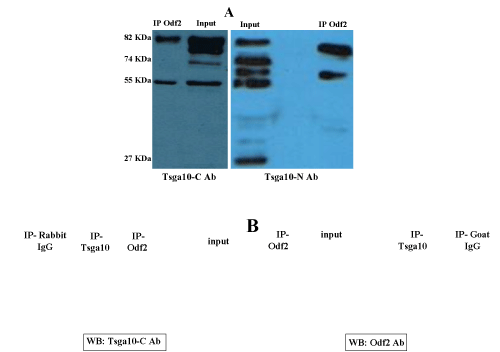
| Figure 2:Identification of ODF2 interaction with full length and 55-KDa fragment of TSGA10 by co-immunoprecipitation. A) Total protein from rat sperm was extracted (in the buffer containing 1% SDS, and 2% DTT), then ODF component was separated and collected via sucrose gradient subjected to immunoprecipitation (IP) followed by Western blot analysis (WB) using antibodies against TSGA10 N- and C-termini. Immunoblots from left to right: panel 1, IP: performed using the anti-ODF2 antibody, WB: anti-TSGA10-C antibody; panel 2, IP: goat anti-ODF2 antibody, WB: rabbit anti-TSGA10-N antibody. Apart from full-length 82-KDa TSGA10 and its spliced form (74 KDa) protein components, different sized proteins (27 KDa and 55KDa) are recognised by antibodies against the N and C-termini, respectively. ODF2 could only bind to full-length as well as its C-terminal 55-KDa component; B) Translated products from in vitro translation experiment (according to the protocol mentioned in the “Materials and Methods”), were used to examine TSGA10 and ODF2 interaction. Two panels of direct and reverse IP followed by WB, showed association of full-length TSGA10 (82 KDa) and ODF2 (84 KDa) proteins. In each IP, relevant IgG was also used as negative control to rule out any non-specific binding of protein to IgG. Left panel, IP: performed using the goat anti-ODF2 antibody, WB: anti-TSGA10-C antibody; Right panel, IP: anti-TSGA10, WB: anti-ODF2 antibody. |