 |
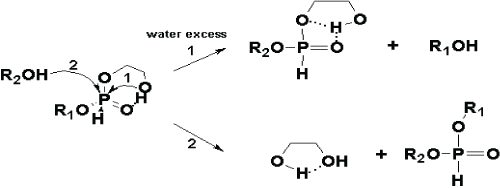
| Figure 5a: Scheme of the mono-ol leaving (pathway 1) and diol leaving (pathway 2), accelerated in the presence of the vicinal hydroxyl group, during the reaction of alcoholysis of H-phosphonate diester. In the presence of water excess (pathway 1), due to the impairing of the intramolecular hydrogen bond between the cis-vicinal 2-OH group and the phosphonyl oxygen atom, by the creation of intermolecular hydrogen bonds (between the cis-vicinal 2-OH group and H2O from the one side, and between the phosphonyl oxygen atom and H2O from the another side) the nucleophilicity of the cis-vicinal 2-OH group is increased and leads to the intramolecular nucleophile attack, supported by the mono-ol (ester alcohol) leaving and following with the ring opening by the intermolecular nucleophile attack by another alcohol molecule. In this case the cisvicinal 2-OH group plays a role as nucleophile catalyst. Since the reaction proceeds in aprotic solvent (pathway 2), due to the electrophile assistance of the cis-vicinal 2-OH group by its intramolecular hydrogen bonding with the phosphonyl oxygen atom, the electrophilicity of the phosphorus atom bonded with latter and its susceptibility to the intermolecular nucleophile attack is increased, which cause the ester exchange (transesterification) reaction, accompanied with the 1,2-diol leaving [45]. In that case the cisvicinal 2-OH group plays a role as electrophile catalyst. |