 |
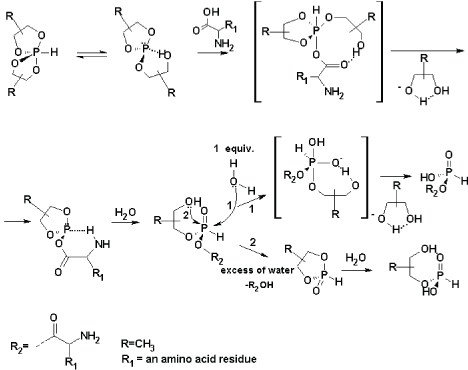
| Figure 6b: Scheme, representing the attack from the nucleophile molecule on phosphonyl phosphorus atom, which is possible in the presence of water. Depending on the water content the reaction is divided with the participation of the amino acid (R2), or 1, 2-propylene glycol as nucleophuges. In both cases it is accompanied by the assistance of the 2-OH group in the beta-hydroxy ethyl ester of H-phosphonic acid derivative [45]. In the presence of 1 equiv. H2O (pathway 1, after the formation of 2-hydroxy propyl amino acyl phosphonate, R2=amino acid residue), this water molecule acts as a nucleophile with formation of the amino acyl phosphonate and leaving of 1, 2-propylene glycol as a nucleophuge. In this case an intramolecular hydrogen bond, created between the 2-OH group and phosphonyl oxygen atom increases the electrophilicity of the phosphonyl phosphorus atom and its susceptibility to the intermolecular nucleophile attack. In the second case (pathway 2, after the formation of 2-hydroxy propyl amino acyl phosphonate), due to the large amount of H2O, the intramolecular hydrogen bond between the 2-OH group and phosphonyl oxygen atom is broken (because of impairing of the intramolecular and creation of intermolecular hydrogen bonds between the molecules of water and the 2-OH group from the one side and between the molecules of water and the phosphonyl oxygen atom from another. This leads to increase of the nucleophile attack from the 2-OH group on the phosphonyl phosphorus atom and leaving of the amino acyl group, and causes the formation of alkylene H-phosphonate (2-oxo 1, 3-dioxa 2-H 2-phospholane), which in the presence of water molecule is hydrolyzed to 2-hydroxyalkyl H-phosphonate. Full studies, concerning the experiments of carrying out of reaction in the desired direction, depending on a variety of water content are included in the forthcoming manuscript and the results will be published elsewhere. |