 |
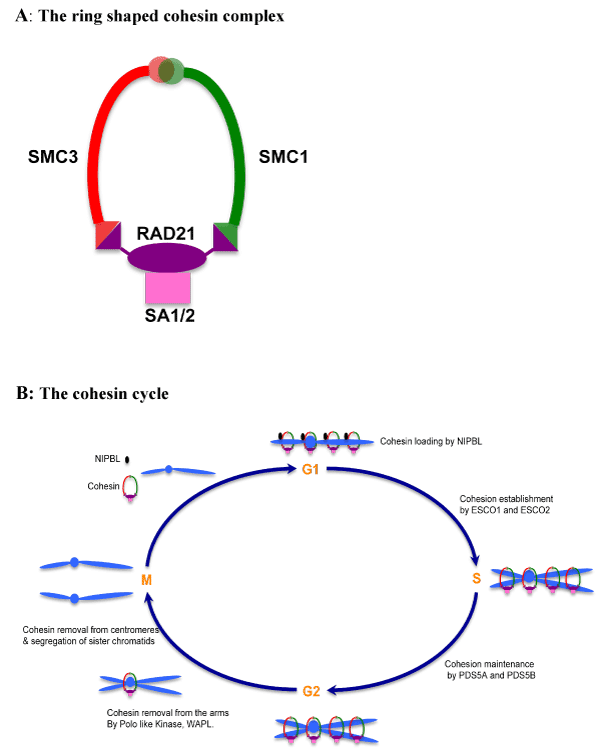
| Figure 1: Cohesin and the cell cycle. (A). Cohesin is a ring-shaped complex. Cohesin is composed of four subunits. Smc1 and Smc3 fold back on themselves by anti-parallel coiled-coil interactions to yield molecules with a ‘hinge’ domain at one end and a globular ATPase ‘head’ at the other. Smc1 and Smc3 interact via the hinge domain, whereas the Smc heads are connected by the α-kleisin Scc1/Rad21. The fourth subunit is called Scc3/ SA (either SA1 or SA2 in vertebrate somatic cells) and interacts with the central region of Scc1/Rad21. (B). Regulation of cohesin during cell cycle. Cohesin loading is mediated by NIPBL. It occurs in G1 in yeast or at the end of telophase of the previous cell cycle in mammalian cells. Cohesion is established by ESCO1 and ESCO2 mediated acetylation during S phase and is maintained in G2 by PDS5A and PDS5B. Cohesin dissolution begins at chromosome arms in prophase at first via phosphorylation by Polo-like kinase 1 and WAPL activity, whereas most pools of cohesin remain protected at centromeric regions by SGO1. Finally, cohesin is completely removed from chromatin by Separase mediated proteolysis. |