 |
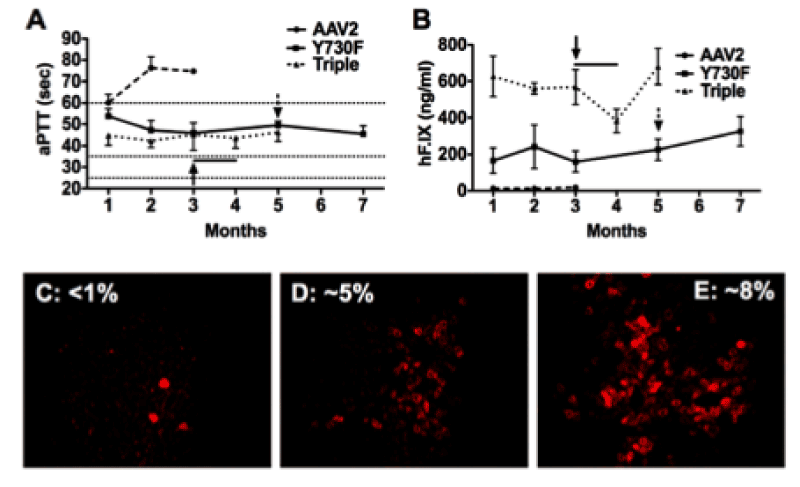
| Figure 2: F.IX -/- C3H/HeJ mice (n=4/vector) were injected into the tail vein with 2x1011 vgs of AAV-ApoE/hAAT-hF.IX packaged into WT AAV-2 (dashed line), or AAV2-Y730F (solid line) or AAV2-Y730+500+444F (dotted line) capsids. Resulting systemic hF.IX expression (A) and coagulation times (aPTT) (B) are shown. Each line represents average SD. Horizontal lines in A mark the range of aPTTs for normal mouse plasma (25-35 sec) or untreated hemophilia B mouse plasma (>60 sec). Y-F vector-treated mice were challenged with subcutaneous hF.IX/CFA (arrow) or weekly intravenous hF.IX (arrow + line). C-E. Immunostain for hF.IX expressing hepatocytes 3-7 months after gene transfer with WT (C), Y730F (D), or Y730+500+444F (E) capsid vectors. Average percent of hF.IX positive hepatocytes are indicated. |