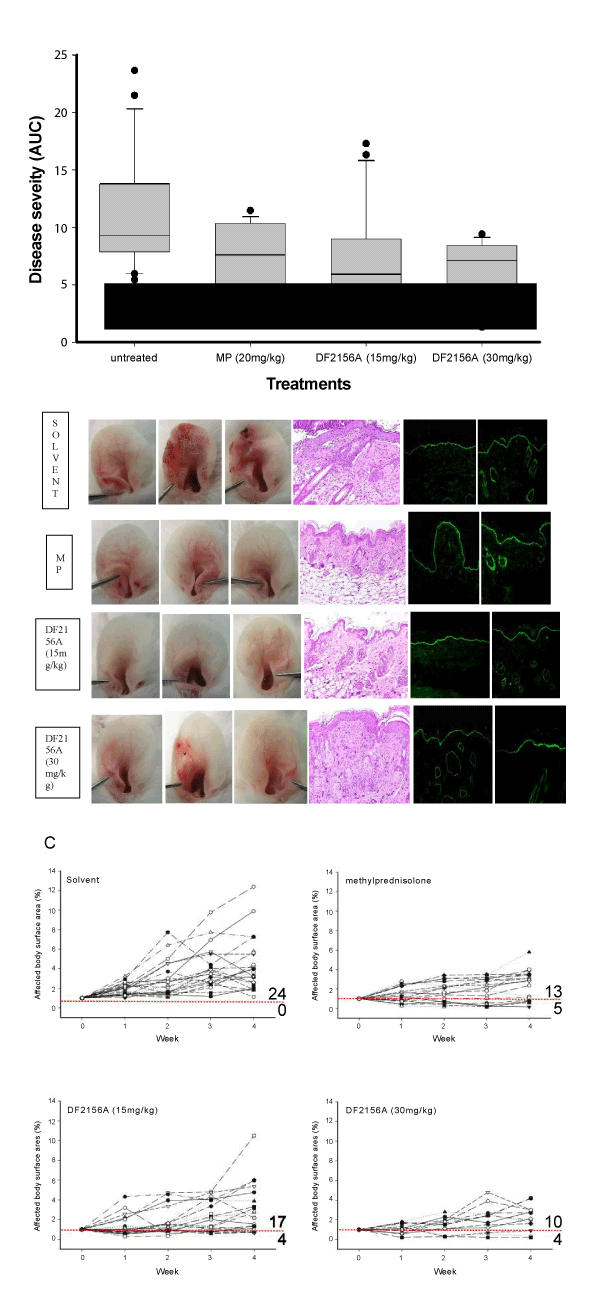
A. Cumulative severity of skin disease in the indicated groups during the 4-week observation period (untreated (saline), n=24; DF2156A (15 mg/kg), n=21; DF2156A (30 mg/kg), n=14; methylprednisolone (MP), n=18). Due to the non-parametric distribution of the numbers, data is presented as median (black line), 75-percentile (box) and 95-percentile (error bars). Numbers outside the 95-percentile are indicated as dots. *indicates statistic significance (ANOVA on Ranks followed by Dunn’s Method for multiple comparisons versus untreated).
B. Representative clinical presentations, H&E-stained skin specimen (ear), and IgG and C3 deposits at the dermal-epidermal junction by direct immunofluorescence microscopy (counterstained with DAPI); skin specimens were obtained at the end of the 4-week treatment period. While MP-treated animals showed a significant reduction in the dermal inflammatory infiltrate, a not significant trend towards a lower infiltration was observed in DF2156A (15 mg/kg)-treated mice. Scale bars at the bottom indicate the original magnification.
C. Relative disease activity (in relation to the affected body surface area at the time of inclusion) of individual mice during the 4-week treatment period. Scores below 1 indicate improvement of experimental EBA, which is indicated by the red dotted line. Compared to untreated mice, scores in all treatment groups were lower at the end of the observation period. While skin disease increased in all 24 control mice, 5/18 (28%) of MP-treated, 4/21 (19%) of DF2156A (15mg/kg) - and 4/14 (29%) of DF2156A (30mg/kg)- treated mice showed a decreased disease severity at the end of the observation period.