 |
|---|
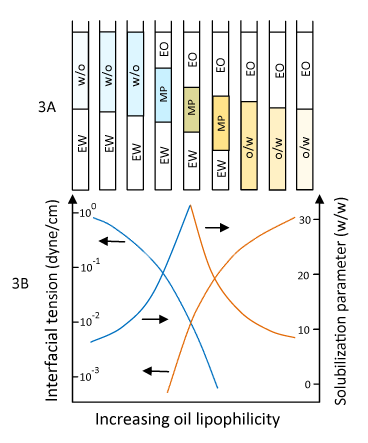
| Figure 3: Changes in phase behavior (3A), solubilization, and interfacial tension (3B) in a microemulsion system with increasing oil lipophilicity. w/o: Water-inoil microemulsion; o/w: Oil-in-water microemulsion; MP: Middle phase (can be w/o, o/w, or bicontinuous); Solubilization parameter is the ratio of the internal phase to the surfactant concentration. Increasing oil lipophilicity can be made by increasing the alkane carbon number (ACN) of hydrocarbon (for example from hexane to hexadecane). From left to right, a transition is seen from w/o microemulsion in equilibrium with excess water (EW), to MP in equilibrium with both EW and excess oil (EO), to o/w microemulsion in equilibrium with EO. In the w/o region, the solubilization of water in oil increases from left to right. The solubilization is maximal in the three phase region. In the o/w region, the solubilization of the oil decreases further from the three phase region and toward higher ACN. Other convenient variables that can be changed include temperature (for nonionic surfactant in particular), salt concentration, etc. Maximum solubilizations occur near and at the transition point: w/o → MP region → o/w (3A), where the lowest interfacial tensions also occur (3B). |