 |
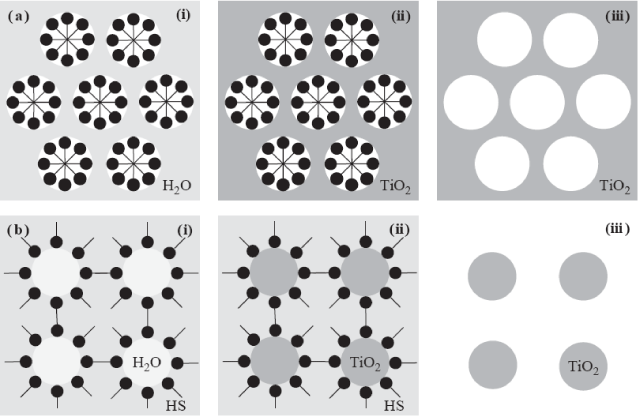
| Figure 5: Synthesis approaches of engineered TiO2 via sol-gel method employing surfactant self assembly as (a)aporetemplate and (b)particle growth template (a)Synthesis of TiO2 with mesoporous inorganic network: (i)surfactant molecules are self-organized in water-rich environment forming surfact and the head group outside towards water molecules and its tail group inside free from water, (ii)titanium alkoxide precursor ishydrolyzed and condensed to form TiO2 inorganic network around the self-assembled surfactant, forming a surfactant organic template-embedded TiO2 inorganic matrix and (iii)porousTiO2 inorganic network is formed after removal of the organic template by thermal treatment or organic extraction. (b)Synthesis of TiO2 nanoparticles: (i)surfactant molecules are self-organized in water-poor environment (bulk hydrophobic solvent(HS) with small portion of water) forming surfactant head group inside towards water molecules and its tail group outside towards HS, (ii)titanium alkoxide precursor is hydrolyzed and condensed to form TiO2 in organic network in the waterphase, inside of self-assembled surfactant, forming TiO2 inorganic core/ surfactant organic shell structure and (iii)well-defined TiO2 nanoparticles are formed after removal of the organic template [61]. |