 |
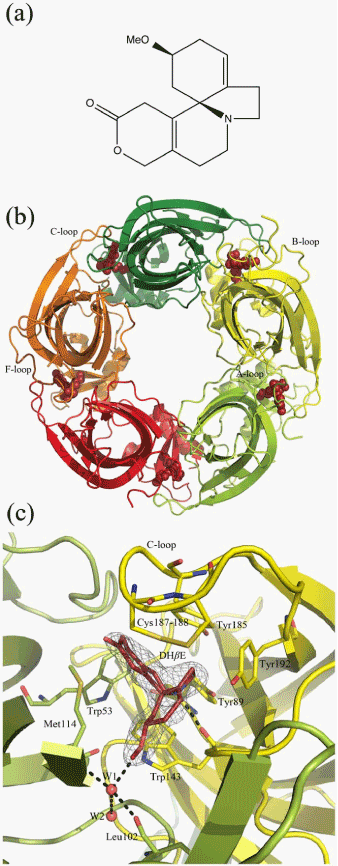
| Figure 3: Structure of AChBP from Lymnaeastagnalis (Ls-AChBP) complexed with the competitive antagonist dihydro-β-erythroidine (DHβE) (modified from [42] ). (a) Structure of DHβE. (b) Complex viewed along the five-fold symmetry axis. The five subunits are shown in different colors and DHβE in red spheres. (c) Interfacial binding pocket formed by the highly conserved aromatic residues Tyr89, Trp143, Tyr185, and Tyr192 from the principal side of the interface (yellow) and Trp53 from the complementary side (limon). DHβE is shown in red, and hydrogen bonds between DHβE and its surroundings are shown as stippled lines. |