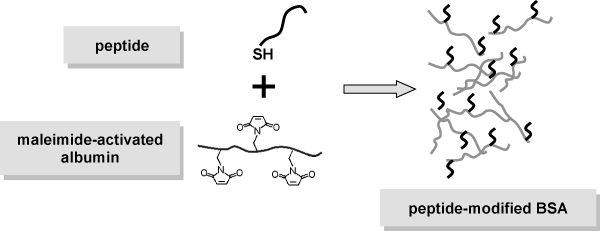
Peptides were synthesized with a short spacer and a free SH-group at the N-terminus. BSA was activated by reaction of 3-(maleimido)-propionic acid N-hydroxysuccinimde ester, attaching a maleimide group to the side chains of the lysins in BSA. The peptide was covalently coupled to BSA by nucleophilic addition of the SH-group to the maleimide double bond (Michael reaction).